- 480.44 KB
- 2022-04-22 11:19:54 发布
- 1、本文档共5页,可阅读全部内容。
- 2、本文档内容版权归属内容提供方,所产生的收益全部归内容提供方所有。如果您对本文有版权争议,可选择认领,认领后既往收益都归您。
- 3、本文档由用户上传,本站不保证质量和数量令人满意,可能有诸多瑕疵,付费之前,请仔细先通过免费阅读内容等途径辨别内容交易风险。如存在严重挂羊头卖狗肉之情形,可联系本站下载客服投诉处理。
- 文档侵权举报电话:19940600175。
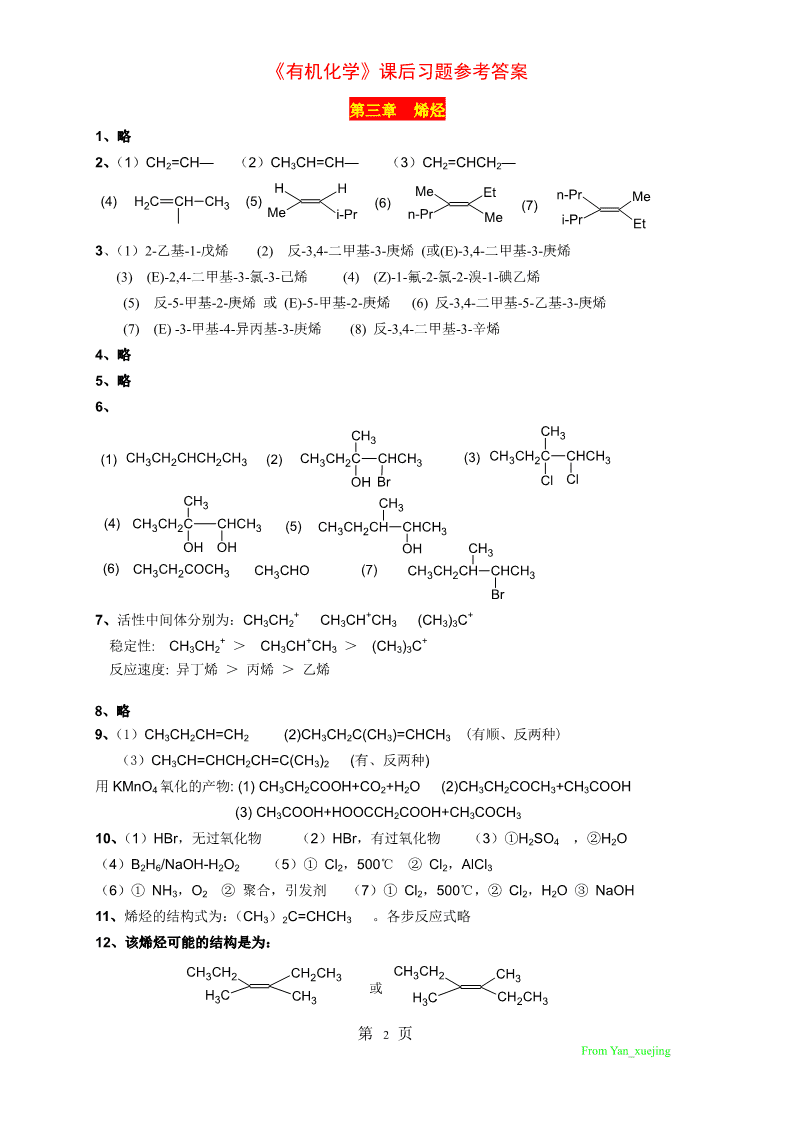
'《有机化学》课后习题参考答案徐寿昌编《有机化学》第二版习题参考答案第二章烷烃1、用系统命名法命名下列化合物(1)2,3,3,4-四甲基戊烷(2)3-甲基-4-异丙基庚烷(3)3,3,-二甲基戊烷(4)2,6-二甲基-3,6-二乙基辛烷(5)2,5-二甲基庚烷(6)2-甲基-3-乙基己烷(7)2,2,4-三甲基戊烷(8)2-甲基-3-乙基庚烷2、试写出下列化合物的结构式(1)(CH3)3CC(CH2)2CH2CH3(2)(CH3)2CHCH(CH3)CH2CH2CH2CH3(3)(CH3)3CCH2CH(CH3)2(4)(CH3)2CHCH2C(CH3)(C2H5)CH2CH2CH3(5)(CH3)2CHCH(C2H5)CH2CH2CH3(6)CH3CH2CH(C2H5)2(7)(CH3)2CHCH(CH3)CH2CH3(8)CH3CH(CH3)CH2CH(C2H5)C(CH3)33、略4、下列各化合物的系统命名对吗?如有错,指出错在哪里?试正确命名之。均有错,正确命名如下:(1)3-甲基戊烷(2)2,4-二甲基己烷(3)3-甲基十一烷(4)4-异丙基辛烷(5)4,4-二甲基辛烷(6)2,2,4-三甲基己烷5、(3)>(2)>(5)>(1)>(4)6、略7、用纽曼投影式写出1,2-二溴乙烷最稳定及最不稳定的构象,并写出该构象的名称。BrBrBrHHHHHHHHBr交叉式最稳定重叠式最不稳定8、构象异构(1),(3)构造异构(4),(5)等同)2),(6)9、分子量为72的烷烃是戊烷及其异构体(1)C(CH3)4(2)CH3CH2CH2CH2CH3(3)CH3CH(CH3)CH2CH3(4)同(1)10、分子量为86的烷烃是己烷及其异构体(1)(CH3)2CHCH(CH3)CH3(2)CH3CH2CH2CH2CH2CH3,(CH3)3CCH2CH3(3)CH3CH2CH(CH3)CH2CH3(4)CH3CH2CH2CH(CH3)214、(4)>(2)>(3)>(1)第1页FromYan_xuejing
《有机化学》课后习题参考答案第三章烯烃1、略2、(1)CH2=CH—(2)CH3CH=CH—(3)CH2=CHCH2—HHMeEtn-Pr(4)H2CCHCH3(5)(6)(7)MeMei-Prn-PrMei-PrEt3、(1)2-乙基-1-戊烯(2)反-3,4-二甲基-3-庚烯(或(E)-3,4-二甲基-3-庚烯(3)(E)-2,4-二甲基-3-氯-3-己烯(4)(Z)-1-氟-2-氯-2-溴-1-碘乙烯(5)反-5-甲基-2-庚烯或(E)-5-甲基-2-庚烯(6)反-3,4-二甲基-5-乙基-3-庚烯(7)(E)-3-甲基-4-异丙基-3-庚烯(8)反-3,4-二甲基-3-辛烯4、略5、略6、CHCH33(1)CH3CH2CHCH2CH3(2)CH3CH2CCHCH3(3)CH3CH2CCHCH3OHBrClClCH3CH3(4)CH3CH2CCHCH3(5)CH3CH2CHCHCH3OHOHOHCH3(6)CH3CH2COCH3CH3CHO(7)CH3CH2CHCHCH3Br+++7、活性中间体分别为:CH3CH2CH3CHCH3(CH3)3C+++稳定性:CH3CH2>CH3CHCH3>(CH3)3C反应速度:异丁烯>丙烯>乙烯8、略9、(1)CH3CH2CH=CH2(2)CH3CH2C(CH3)=CHCH3(有顺、反两种)(3)CH3CH=CHCH2CH=C(CH3)2(有、反两种)用KMnO4氧化的产物:(1)CH3CH2COOH+CO2+H2O(2)CH3CH2COCH3+CH3COOH(3)CH3COOH+HOOCCH2COOH+CH3COCH310、(1)HBr,无过氧化物(2)HBr,有过氧化物(3)①H2SO4,②H2O(4)B2H6/NaOH-H2O2(5)①Cl2,500℃②Cl2,AlCl3(6)①NH3,O2②聚合,引发剂(7)①Cl2,500℃,②Cl2,H2O③NaOH11、烯烃的结构式为:(CH3)2C=CHCH3。各步反应式略12、该烯烃可能的结构是为:CH3CH2CH2CH3CH3CH2CH3或H3CCH3H3CCH2CH3第2页FromYan_xuejing
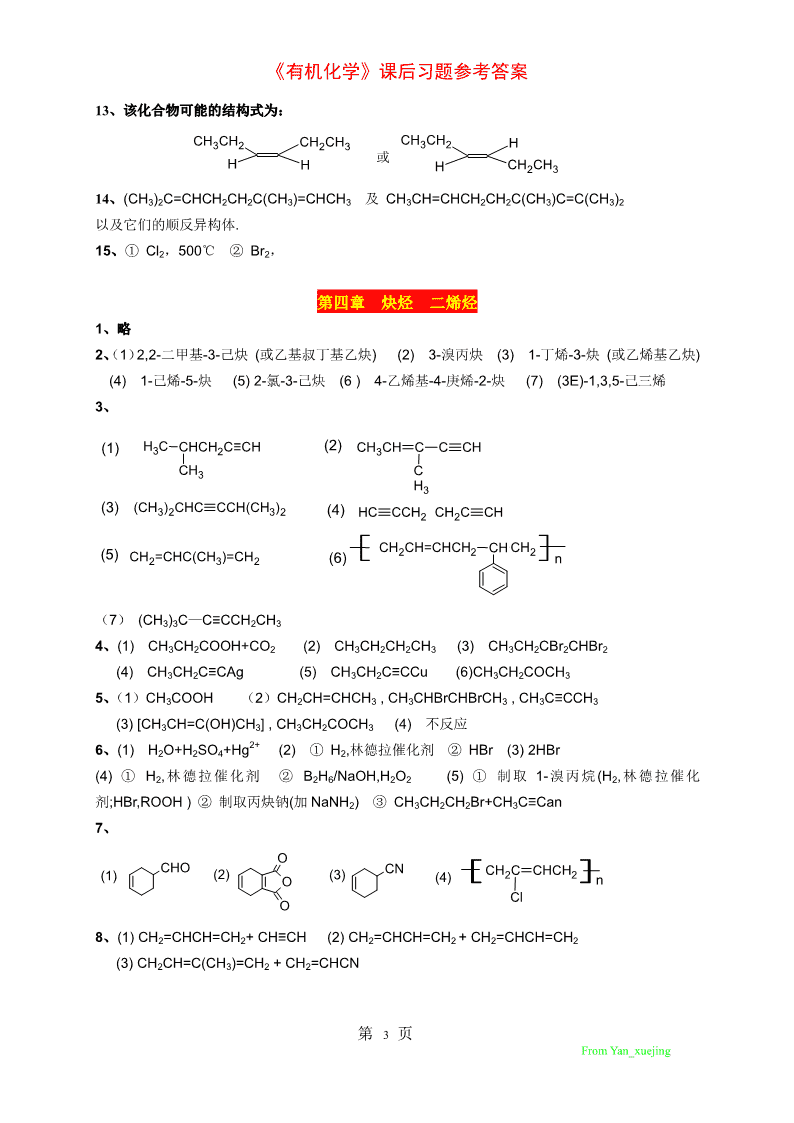
《有机化学》课后习题参考答案13、该化合物可能的结构式为:CH3CH2CH2CH3CH3CH2H或HHHCH2CH314、(CH3)2C=CHCH2CH2C(CH3)=CHCH3及CH3CH=CHCH2CH2C(CH3)C=C(CH3)2以及它们的顺反异构体.15、①Cl2,500℃②Br2,第四章炔烃二烯烃1、略2、(1)2,2-二甲基-3-己炔(或乙基叔丁基乙炔)(2)3-溴丙炔(3)1-丁烯-3-炔(或乙烯基乙炔)(4)1-己烯-5-炔(5)2-氯-3-己炔(6)4-乙烯基-4-庚烯-2-炔(7)(3E)-1,3,5-己三烯3、(1)H3CCHCH2C≡CH(2)CH3CHCCCHCH3CH3(3)(CH3)2CHCCCH(CH3)2(4)HCCCH2CH2CCH(5)(6)CH2CH=CHCH2CHCH2CH2=CHC(CH3)=CH2n(7)(CH3)3C—C≡CCH2CH34、(1)CH3CH2COOH+CO2(2)CH3CH2CH2CH3(3)CH3CH2CBr2CHBr2(4)CH3CH2C≡CAg(5)CH3CH2C≡CCu(6)CH3CH2COCH35、(1)CH3COOH(2)CH2CH=CHCH3,CH3CHBrCHBrCH3,CH3C≡CCH3(3)[CH3CH=C(OH)CH3],CH3CH2COCH3(4)不反应2+6、(1)H2O+H2SO4+Hg(2)①H2,林德拉催化剂②HBr(3)2HBr(4)①H2,林德拉催化剂②B2H6/NaOH,H2O2(5)①制取1-溴丙烷(H2,林德拉催化剂;HBr,ROOH)②制取丙炔钠(加NaNH2)③CH3CH2CH2Br+CH3C≡Can7、OCHOCNCH2CCHCH2(1)(2)O(3)(4)nClO8、(1)CH2=CHCH=CH2+CH≡CH(2)CH2=CHCH=CH2+CH2=CHCH=CH2(3)CH2CH=C(CH3)=CH2+CH2=CHCN第3页FromYan_xuejing
《有机化学》课后习题参考答案9、(1)①①H2,林德拉催化剂HCCHCH3CH2Br(A)②HBrNaNH2HCCHHCCNa(B)液氨②(A)+(B)H2O,H2SO4,HgSO4还原CHCCCH33TM(2)可用(1)中制取的1-丁炔+2HCl(3)可用1-丁炔+H2,林德拉催化剂,再加HBr+ROOH10、NaNH2(1)HC≡CHHC≡CNaCHCHCH=CHHBr,ROORCHCHCHCHBrHC≡CNa3223222H2SO4,H2OCH3CH2CH2CH2C≡CHCH3CH2CH2CH2COCH3HgSO4500℃(2)CHCH=CHHC≡CHNaCH≡CNa32ClCH2CH=CH2Cl2CH2CHCl2+CH2ClCH≡CNaCH2C≡CHCH2COCH3CHCH2H2O,Hg2SO4,H2SO42NH3,O2(3)CH3CH=CH2CH2=CHCN催化剂ClCNCNCNCl2+Cl11、(1)①Br2/CCl4②银氨溶液(2)银氨溶液12、(1)通入硝酸银的氨溶液中,乙炔迅速生成乙炔银沉淀而除去.(2)用林德拉催化剂使乙炔氢化为乙烯.13、(1)1,2-加成快,因为1,2-加成活化能低于1,4-加成活化能.(2)1,4-加成产物比1,2-加成产物的能量低,更稳定.14、可利用”动力学控制”和”热力学控制”理论解释.15、此烃为:CH2=CHCH=CHCH=CH2(有顺反异构体)第五章脂环烃1、(1)1-甲基-3-异丙基环己烯(2)1-甲基-4-乙烯基-1,3-环己二烯(3)1,2-二甲基-4-乙基环戊烷(4)3,7,7-三甲基双环[4.1.0]庚烷(5)1,3,5-环庚三烯(6)5-甲基双环[2.2.2]-2-辛烯(7)螺[2.5]-4-辛烯第4页FromYan_xuejing
《有机化学》课后习题参考答案CH32、(1)(2)CH3(3)CH3CH3(4)CH(5)(6)(7)33、略4、(1)、)2)、(3)无(4)顺、反两种(5)环上顺反两种,双键处顺反两种,共四种(6)全顺、反-1、反-2、反-4共四种5、HCH3HH(1)CH(CH)(2)(3)CH332CH(CH3)2CH(CH3)2HHC2H5CH3HHH(5)(CH3)3CH(4)(CH3)3CHCH36、H2/NiCH3CH2CH2CH3(1)CHBr2CHCHBrCHCHBr3322HICH3CH(I)CH2CH3CH3H2/NiBrCH3Br2CH3Br(2)HCO3OO3Zn/HOO2CH3CO(CH2)4CHOHBrH3CBrCH=CHCNCN2BrBr2Br2(3)BrBrCH2=CHCOOC2H5COOC2H5第5页FromYan_xuejing
《有机化学》课后习题参考答案CH3CH3光(4)+Br2Br+HBr7、该二聚体的结构为:(反应式略)8、CH3H3CCH3CH=CH2CHH3CCH339、(A)(B)CH3CH2C≡CH(C)CH3C≡CCH3第六章单环芳烃1、略2、NO2NO2C2H5COOH(1)(2)(3)(4)NO2H5C2C2H5BrOHCH3CH2ClO2NNO2OHSO3H(5)(6)(7)(8)NO2IClO2NNO23、(1)叔丁苯(2)对氯甲烷(3)对硝基乙苯(4)苄醇(5)苯磺酰氯(6)2,4-二硝基苯甲酸(7)对十二烷基苯磺酸钠(8)1-对甲基苯-1-丙烯4、(1)①Br2/CCl4②加浓硫酸(或HNO3+H2SO4)(2)①Ag(NH3)2NO3②Br2/CCl4BrNHCOCH3CH25COOH5、(1)(2)(3)(4)COOHCOOHOCH3COOHOH(5)(6)(7)(8)CHOCH3NO23OHOHOHOHBrCH3(9)(10)(11)(12)CH3CH36、(1)AlCl3ClSO3H第6页FromYan_xuejing
《有机化学》课后习题参考答案COOHC2H5(2)CH3CH=CH2(3)Cl2,光CH(4)27、(1)A错,应得异丙苯;B错,应为氯取代α-H。(2)A错,硝基苯部发生烷基化反应;B错,氧化应得间硝基苯甲酸。(3)A错,-COCH3为间位定位基;B错,应取代环上的氢。8、(1)间二甲苯>对二甲苯>甲苯>苯(2)甲苯>苯>溴苯>硝基苯(3)对二甲苯>甲苯>对甲苯甲酸>对苯二甲酸(4)氯苯>对氯硝基苯>2,4-二硝基氯苯9、只给出提示(反应式略):(1)4-硝基-2-溴苯甲酸:①硝化②溴代(环上)③氧化3-硝基-4-溴苯甲酸:①溴代(环上)②氧化③硝化(2)①溴代(环上)②氧化(3)①氧化②硝化(4)①硝化②溴代(环上)(5)①硝化②氧化(6)①烷基化②氧化③氯代10、只给出提示(反应式略):(1)以甲苯为原料:①Cl2/Fe②Cl2,光(2)以苯为原料:①CH2=CH2,AlC3②Cl2,光③苯,AlCl3(3)以甲苯为原料:①Cl2,光②CH3Cl,AlCl3③甲苯,AlCl3(4)以苯为原料:①CH3CH2COCl,AlC3②Zn-Hg+HClOOV2O5[H](5)OOO2OOOOAlCl3Zn-HgSOCl2+OHClHOOCHOOCOAlCl3ClOCO11、(1)乙苯(2)间二甲苯(3)对二甲苯(4)异丙苯或正丙苯(5)间甲乙苯(6)均三甲苯12、两种:连三溴苯;三种:偏三溴苯;一种一元硝基化合物:均三溴苯。13、OCHCH3CH3COOH3ABCDEOC≡CHC2H5COOHO14、15、略第7页FromYan_xuejing
《有机化学》课后习题参考答案第七章立体化学1、(1)3(2)4(3)2(4)8(5)0(6)4(7)0(8)2(9)32、略3、(1)(4)(5)为手性分子4、对映体(1)(5)相同(2)(6)非对映体(3)(4)5、********HOC2H5HH6、(1)ClH(S)(2)BrF(S)(3)H5C2C(CH3)2Br(S)BrClDCH3CH3CH3ClHHOH(4)NH2(R)(5)(2R,3R)(6)HOH(2S,3R)HClHCH3CH2OH7、(4)为内消旋体CH2CH2CH3CH3CHNH22BrOH(1)HOH(2)(3)HOHHBrCH3CH2OHClHC2H5CH2OHCH3HOHBr(4)HOH(5)(6)CH3OHCH3CHCHOH252H8、C2H5C2H5C2H5C2H5(1)H3CH(2)HCH3(3)H3CCl(4)HCH2ClCH2CH2ClCH2CH2ClC2H5C2H5C2H5C2H5C2H5C2H5(5)H3CH(6)HCH3(7)H3CH(8)HCH3HClClHClHHClCH3CH3CH3CH3(1)(2);(5)(6);(7)(8)为对映体(5)(7);(5)(8);(6)(7);(6)(8)为非对映体9、(A)CH3CH2CH(OH)CH=CH2(B)CH3CH2CH(OH)CH2CH310、(A)CH2=CHCH2CH(CH3)CH2CH3(B)CH3CH=CHCH(CH3)CH2CH310、(A)CH2=CHCH2CH(CH3)CH2CH3(B)CH3CH=CHCH(CH3)CH2CH3第8页FromYan_xuejing
《有机化学》课后习题参考答案(C)CH3CHCH2CH(CH3)CH2CH311、H3CH3CBrH3CCH3HBrCH2+BrBrCH3BrBr(A)(B)(C)BrH3C(B)(CH3)3COK+H2C≡≡(A)CH2CH3BrCH3H3C(CH3)3COK(C)H2C+CH(外消旋体)2BrBr(CH3)3COK(A)①O2H2CCH2OO+2HCHO②Zn,H2O(D)第九章卤代烃1、(1)1,4-二氯丁烷(2)2-甲基-3-氯-6-溴-1,4-己二烯(3)(E)-2-氯-3-己烯(4)2-甲基-3-乙基-4-溴戊烷(5)对氯溴苯(6)3-氯环己烯(7)四氟乙烯(8)4-甲基-1-溴-1-环己烯2、(1)CH2=CHCH2Cl(3)CH3C≡CCH(CH3)CH2Cl(6)Cl2C=CH2(7)CF2Cl2(8)CHCl3(2)(4)(5)CH2BrBrCH2CH2Br3、(1)CH3CHBrCH3CH3CH(CN)CH3(2)CH3CH2CH2BrCH3CH2CH2OH(3)ClCH2CH=CH2ClCH2CH(OH)CH2ClClBrI(4)(5)Cl(6)CH3CH(CH3)CHClCH3CH3CH(CH3)CH(NH2)CH3(7)(CH3)2C=CH2(8)PBr3CH3CH(ONO2)CH3+AgBr↓(9)CH3CH2CH2CH2C≡CMgBr+C2H5(10)ClCH=CHCH2OCOCH3(11)Cl2CHCHCl2Cl2C=CHClCH2CNCH2NH2CH2OC2H5CH2ICH2OH(12)第9页FromYan_xuejing
《有机化学》课后习题参考答案4、(只给出主要产物,反应式略)(1)CH3CH2CH2CH2OH(2)CH3CH2CH=CH2(3)A:CH3CH2CH2CH2MgBrB:CH3CH2CH2CH3+HC≡CMgBr(4)CH3CH2CH2CH2I+NaBr(5)CH3CH2CH2CH2NH2(6)CH3CH2CH2CH2CN(7)CH3CH2CH2CH2ONO2+AgBr↓(8)CH3C≡CCH2CH2CH2CH3(9)CH3(CH2)6CH3(10)CH3CH2CH2CH2N(CH3)25、(1)加AgNO3(醇),有白色沉淀析出,反应速度:CH2=CHCH2Cl>CH3CH2CH2Cl>CH3CH=CHCl(几乎不反应)(2)加AgNO3(醇),有白色沉淀析出,反应速度:苄氯>氯代环己烷>氯苯(不反应)(3)加AgNO3(醇),分别有白色、蛋黄色、黄色沉淀生成(4)试剂、现象同(2),反应速度:苄氯>1-苯基-2-氯乙烷>氯苯(不反应)6、(1)a:(CH3)3CBr>CH2CH2CHBrCH3>CH3CH2CH2CH2Br∨∨b:CHBrCH3CH2BrCH2CH2Br(2)a:CH3CH2CH2Br>(CH3)2CHCH2Br>(CH3)3CCH2Brb:CH3CH2CH2CH2Br>CH3CH2CHBrCH3>(CH3)3CBr7、(1)(CH3)2CBrCH2CH3>(CH3)2CHCHBrCH3>(CH3)2CHCH2CH2BrCHBrCH3CHBrCH3CHBrCH3CHBrCH3∨∨∨(2)OCH3CH3NO28、(1)CH3CH2CH2CH2Br反应较快。因为甲基支链的空间阻碍降低了SN2反应速度。(2)(CH3)3CBr反应较快,为SN1反应。(CH3)2CHBr首先进行SN2反应,但谁为弱的亲核试剂,故反应较慢。(3)-SH反应快于-OH,因为S的亲核性大于O。(4)(CH3)2CHCH2Br快,因为Br比Cl易离去。9、(1)SN2(2)SN1(3)SN2(4)SN1(5)SN1(6)SN2(7)SN210、(1)(A)错,溴应加在第一个碳原子上。(B)错,-OH中活泼H会与格氏试剂反应。(2)(A)错,HCl无过氧化物效应。(B)错,叔卤烷遇-CN易消除。(3)(B)错,与苯环相连的溴不活泼,不易水解。(4)错,应消除与苯环相邻碳上的氢。11、只给出提示(反应式略):(1)①KOH(醇)②HBr过氧化物(2)①KOH(醇)②Cl2,500℃③[H]第10页FromYan_xuejing
《有机化学》课后习题参考答案(3)①KOH(醇)②Cl2③2KOH(醇)④2HCl(4)①KOH(醇)②Cl2,500℃③Cl2(5)①Cl2,500℃②HOCl③Ca(OH)2,100℃(6)①Cl2,500℃②Cl2③KOH(醇)④KOH,H2O⑤KOH(醇)(7)①KOH(醇)②HCl(8)①KOH(醇)②Br2(9)①Cl2②H2/Ni③NaCN(10)1,1-二氯乙烯:①HCl②Cl2③KOH(醇)三氯乙烯:①2Cl2②KOH(醇)(11)①KOH(醇)②Cl2,500℃③KOH,H2O(12)①HCHO/ZnCl2+HCl②KOH,H2O(13)①KOH(醇)②Br2③2KOH(醇)④Na,液氨⑤CH3CH2CH2Br12、略13、(反应式略)ABCH3C14、(反应式略)A:CH2=CHCH(CH3)CH2CH3B;CH3CH2CH(CH3)CH2CH3(无旋光性)15、(反应式略)A:CH3CH(Br)CH=CH2B:CH3CHBrCHBrCH2BrC:CH3CH(OH)CH=CH2D:CH3CH=CHCH2OHE:CH3CH(OH)CH2CH3F:CH3CH2CH2CH2OHwsy024第十章醇和醚1、(1)2-戊醇2°(2)2-甲基-2-丙醇3°(3)3,5-二甲基-3-己醇3°(4)4-甲基-2-己醇2°(5)1-丁醇1°(6)1,3-丙二醇1°(7)2-丙醇2°(8)1-苯基-1-乙醇2°(9)(E)-2-壬烯-5-醇2°(10)2-环己烯醇2°(11)3-氯-1-丙醇1°2、(2)>(3)>(1)3、按形成氢键多少和可否形成氢键排列:(4)>(2)>(1)>(3)>(5)4、(1)①Br2②AgNO3(醇)(2)用卢卡试剂(ZnCl+HCl),反应速度:3°>2°>1°(3)用卢卡试剂(ZnCl+HCl),α-苯乙醇反应快。5、分别生成:3-苯基-2-丁醇和2-甲基戊醇6、只给出主要产物(1)CH3CH=C(CH3)2(2)(CH3)2C=CHCH2OH(3)C6H5-CH=CHCH3第11页FromYan_xuejing
《有机化学》课后习题参考答案(4)C6H5-CH=CHCH(CH3)2(5)CH3CH=C(CH3)C(CH3)=CHCH37、(1)对甲基苄醇>苄醇>对硝基苄醇(2)α-苯基乙醇>苄醇>β-苯基乙醇8、提示:在反应过程中,形成烯丙基正离子,因而生成两种产物。9、略10、(反应式略)(1)CH3CH2CH2MgBr+CH3CHO或CH3MgBr+CH3CH2CH2CHO(2)CH3MgBr+CH3COCH2CH3或CH3CH2MgBr+CH3COCH3(3)CH3CH2MgBr+C6H5-CHO或C6H5-MgBr+CH3CH2CHO(4)CH3MgBr+C6H5-COCH3或C6H5-MgBr+CH3COCH3[O]11、(1)CH3OHHCHOPBr3MgCH3CHCH2CH3CH3CH(OH)CH2CH3CH3CHBrCH2CH3干醚MgBrHCHOHO+CH3CHCH2CH33CH3CHCH2CH3干醚CH2OMgBrCH2OHPBr3Mg(2)CH3CH2CH2OHCH3CH2CH2MgBr干醚CH3[O]CH3CH2CH2MgBrCH2CH(OH)CH3CH3COCH3H3CCH2CH2CH3干醚OMgBr+CH3H3OH3CCH2CH2CH3OH+(3)CH干醚H3OCHCHCHOHHCHO+3CH2MgBr322+CH3CHO+CH3MgBr干醚H3OCH3CH(OH)CH3PBr3Mg(4)(CH3)2CHOH(CH3)2CHMgBr干醚[O](CH3)2CHMgBr(CH3)2CHCHCH(CH3)2(CH3)2CHCH2OH(CH3)2CHCHO干醚OMgBr++(CH)CHCHCH(CH)H△H3O3232(CH3)2CHCH=C(CH3)2OH-H2O(5)①Cl2,500℃②H2O,NaOH③HOCl④H2O,NaOH⑤3HNO3水合氧化CHCOCH(6)CH3CH=CH233CH2CH3CH=CH2CH2CH2BrAlCl3-H2HBrMg+CH2=CH2催化ROOR干醚+CH2CH2MgBrCH+CH2CH2C(OH)(CH3)2H△CH2CH=C(CH3)23COCH3H3O干醚-H2O第12页FromYan_xuejing
《有机化学》课后习题参考答案12、只给出提示(反应式略):(1)①–H2O②HCl(2)①-H2O②直接或间接水合(3)①–H2O②HBr③KOH/醇13、只给出提示(反应式略):(1)①PBr3②Mg/干醚③环氧乙烷④H2O++(2)①CH3CH2CH2Mg,干醚②H3O③–H2O/H,△④硼氢化氧化(3)①C2H5Cl/AlCl3②NaOH③CH3I④CH3CH2CH2COCl/AlCl3⑤LiAlH4+(4)选1,3-丁二烯和3-丁烯-2-酮①双烯合成②CH3Mg③H3O④H2/Ni14、该醇的结构式为:(CH3)2CHCH(OH)CH315、原化合物的结构式为:CH3CH(OH)CH2CH3或CH3CH2CH2CH2OH(反应式略)16、A:(CH3)2CHCHBrCH3B:(CH3)2CHCH(OH)CH3C:(CH3)2C=CHCH3(反应式略)CH3+CH3+17、HCCHOHHHCCH+重排H3CCCH2CH33232-H2OCHCH3CH33+①O-HH3CCCHCH33H3CCOCHCHO+3②Zn/H2OCHCH3318、A:CH3CH2CHBrCH(CH3)2B:CH3CH2CH=C(CH3)2C:CH3CH=CHCH(CH3)2D:CH3CH2CHOE:CH3COCH3(各步反应式略)19、(1)CH3OCH2CH2CH3甲丙醚(甲氧基丙烷)C2H5OC2H5乙醚(乙氧基乙烷)CH3OCH(CH3)2甲异丙醚(2-甲氧基丙烷)(2)CH3OCH2CH2CH2CH3甲丁醚(甲氧基丁烷)CH3OCH(CH3)CH2CH3甲仲丁醚(2-甲氧基丁烷)CH3OCH2CH(CH3)2甲异丁醚(1-甲氧基-2-甲基丙烷)CH3OC(CH3)3甲叔丁醚(2-甲氧基-2-甲基丙烷)CH3CH2OCH2CH2CH3乙丙醚(乙氧基丙烷)CH3CH2OCH(CH3)2乙异丙醚(2-乙氧基丙烷)20、(1)加金属钠,乙醇钠在乙醚中是固体,可分离。(2)①加Ag(NH3)2NO3,1-戊炔有白色沉淀生成,分离,再加稀硝酸可还原为炔。②加金属钠,1-甲氧基-3-戊醇可生成醇钠(固体),分离,再加水可还原为原化合物。21、(只给出主要产物,反应式略)(1)CH3OCH2CH2CH3+NaI(2)CH3CH2OCH(CH3)CH2CH3+NaBr(3)CH3CH2C(CH3)2OCH2CH2CH3+NaCl(4)(CH3)2C=CH2+CH3CH2CH2OH+NaCl第13页FromYan_xuejing
《有机化学》课后习题参考答案NO2(5)OH+CH3I(6)O2NOCH2CH3+OCH2CH322、只给出提示(反应式略):(1)制取乙二醇二甲醚:①乙烯O2/Ag,△②2CH3OH+制取三甘醇二甲醚:①乙烯O2/Ag,△②H2O,H③环氧乙烷④环氧乙烷⑤2CH3OH/H2SO4,△(2)①O2/Ag,△②NH3③环氧乙烷(3)①O2/Cu,加压、△制得甲醇②–H2O(4)①直接或间接水合制异丙醇②–H2O(5)从苯出发:①Cl2/Fe②HNO3+H2SO4③Na2CO3④CH3Br其中CH3OH+HBr→CH3Br+H2O(6)①由CH2=CH2→CH3CH2MgBr②由CH2=CH2→环氧乙烷③由①和②→CH3CH2CH2CH2OH④–H2O23、因分子中含有羟基越多则形成分子间氢键越多,沸点越高。乙二醇二甲醚不能形成分子间的氢键,因而沸点是三者中最低的。24、(1)CH3I,CH3CH2CH2CH2I(2)CH3I,CH3CH(I)CH2CH2CH3(3)CH3I,CH3CH2CH(CH3)CH2I25、该化合物的结构式为:CH3CH2CH2OCH2CH2CH3(有关反应式略)26、此化合物为:CH3(CH2)4-O-CH2CH327、mmolAgI=mmolCH3I化合物C20H21O4N相对分子质量为339,所以11.62mg/235mg(1molAgI)=11.62/235(1molCH3I)mmolCH3I11.62/235==4(此化合物分子中所含OCH3数)mmol化合物4.24/339++28、(1)CH3OH++H3COHCH2CH2OH-HCH3OCH2CH2OHOH-+(2)H3CO+CH3OCH2CH2O+HCH3OCH2CH2OHO第十一章酚和醌1、(1)间甲苯酚(2)4-乙基-1,3-苯二酚(3)2,3-二甲基苯酚(4)2,4,6-三硝基苯酚(5)邻甲氧基苯酚(6)对羟基苯磺酸(7)1-甲基-2-萘酚(8)9-蒽酚(9)1,2,3-苯三酚第14页FromYan_xuejing
《有机化学》课后习题参考答案(10)5-甲基-2-异丙基苯酚(11)5-硝基-1-萘酚(12)β-氯蒽醌OHOHOCH2COOHOHClBrBr2、(1)(2)(3)(4)NO2NH2ClBrOHOHOCOCH3(CH3)3CC(CH3)3SO3Na(5)(6)(7)(8)C(CH)O33OSO3HOHO(9)HO3S(10)OOHOOCH3(11)HOOH(12)CH3NOHOHHCCH33-3FeCl+-3、(1)6+3FeO6+6H+3ClOHOHBrCH3OHONaCH3CH3CH3(2)+Br2/H2O(3)+NaOHBrOHOCOCH3OHOCOCH3CH3CH3CH3CH3(4)+CH3COCl(5)+(CH3CO)2)OOHOHOHCH3O2NCHCH33(6)+HNO3(稀)+(次)(主)NO2OHOHClCHCH33(7)+Cl2(过量)ClOHOHOHCHHOSCHCH3333+(8)+H2SO4(浓)(次)(主)SO3HOHONaOCH3CH3CH3(CH3)2SO4CH3(9)+NaOH第15页FromYan_xuejing
《有机化学》课后习题参考答案OHONaOHCH3+-CH3(10)H或HOHOH2CCH3+HCHO+CH2OH(主)(次)4、①FeCl3②Na5、(1)用氢氧化钠水溶液,苯酚成酚钠溶于水,然后用分液漏斗分离,再酸化。(2)、(3)、(4)同样可用氢氧化钠水溶液将相应得酚分离出来。6、在苯酚分子中引入吸电子基可使酸性增强,其中邻、对位的酸性大于间位,所以酸性由大到小的顺序为:2,4-二硝基苯酚>对硝基苯酚>间硝基苯酚>苯酚7、水杨醇不溶于碳酸氢钠溶液而容于氢氧化钠溶液,酸化后又可析出,且与三氯化铁溶液反应显蓝紫色,故可证明分子中含有酚羟基。当用氢溴酸处理,分子中羟基被溴原子取代,有分层现象出现,证明分子中有醇羟基。8、(2)、(4)能形成分子内氢键,(1)、(3)能形成分子间氢键。+9、(1)以苯为原料:①浓硫酸(磺化)生成间苯三磺酸②NaOH,△(碱熔)③H(2)以苯为原料:①C2H5Cl,AlCl3②浓硫酸(磺化)生成4-乙基-1,3-苯二磺酸+③NaOH,△(碱熔)④H+(3)苯:①磺化,②NaOH,△(碱熔)③H④HNO2(4)由上制得苯酚钠,再加C2H5I即可。(5)①由上得苯酚②Cl2,△③Cl2,△制得2,4-二氯苯酚④NaOH⑤CH2ClCOOH(6)①由上制得苯酚钠②CH3I③硝化NOHOHOH同(3)2(CH3)2C=CH2(H3C)3CC(CH3)3HNO3(H3C)3CC(CH3)3(8)H2SO4NO2+(9)①制取苯酚②磺化→对羟基苯磺酸③Cl2④H1O,H,△(10)①制取苯酚②C2H5Cl,AlCl3③Br2,FeCl310、Cl2HOClCa(OH)2CH3CH=CH2CH2ClCH(OH)CH2ClClCH2CHCH2500℃OOCH3OCH3ClCH2CHCH2OCH3同(6)①磺化ONaOOCH2CHCH2②碱熔OOCH3+H3OOCH2CH(OH)CH2OH11、(1)①磺化,碱熔→间苯二酚钠②CH3I第16页FromYan_xuejing
《有机化学》课后习题参考答案CH2CH2OHNaOHCH2CH2OHCH2CH2Br(2)PCl5OHONaONaO+(3)①磺化,NaOH,△(碱熔),H→对甲苯酚②CH3COCl,AlCl312、该化合物结构式为:OOH(A)或OO(B)或HOOHOOHOCH3OH13、(A)(B)(C)CH3I第十二章醛、酮1、(1)3-甲基戊醛(2)2-甲基-3-戊酮(3)甲基环戊基甲酮(4)间甲氧基苯甲醚(5)3,7-二甲基-6-辛烯醛(6)α-溴代苯乙酮(7)乙基乙烯基甲酮(8)丙醛缩二乙醇(9)环己酮肟(10)2,4-戊二酮(11)丙酮-2,4-二硝基苯腙OHOOCH32、(1)CH3CH=CHCHO(2)C(3)(4)CH2CH2CHOCH3(5)NHN=CH2(6)(CH3)2C=NNHCONH2(7)CH2CH2COCH3(8)CH3CHBrCHO(9)OO(10)CHOOHO3、略4、(1)CH3CH2CH2OH(2)CH3CH2CH(OH)C6H5(3)CH3CH2CH2OH(4)CH3CH2CH(OH)SO3Na(5)CH3CH2CH(OH)CN(6)CH3CH2CH(OH)CH(CH3)CHO(7)CH3CH2CH=C(CH3)CHOO(8)CH3CH2CH2OH(9)CH3CH2CH(10)CH3CHBrCHOO(11)CH3CH2COONH4,Ag(12)CH3CH2CH=NOH(13)NHN=CHCH2CH35、(1)H3CCH=CHCHO+H2O(2)H3CCOONa+H3CCH2OH(3)(5)H3CCH2OH+HCOONa(4)H3CCOOHHOOCCOOH第17页FromYan_xuejing
《有机化学》课后习题参考答案6、(1)COCH3(2)COONa+CHCl3(3)CH2(OH)CH3O2NOMgBrOH(4)CH3CH37、(1)(3)(6)(7)能发生碘仿反应;(1)(2)(4)能与NaHSO3发生加成反应,8、(1)CF3CHO>CH3CHO>CH3COCH3>CH3COCH=CH2(2)ClCH2CHO>BrCH2CHO>CH3CH2CHO>CH2=CHCHO9、(1)加2,4-二硝基苯肼(2)加托伦试剂(3)碘仿反应(4)饱和NaHSO3水溶液(5)2,4-二硝基苯肼(6)碘仿反应10、只给出主要产物,反应式略:OHOHCH3OOH(1)CH3CH2CH2CHCHCHO,CH3CH2CH2CHCHCH2OH(2),,CH2CH3CH2CH3OOC2H5OC2H5(CH3)2CCHO(3)(CH3)2CBrCHO,(CH3)2CBrCH,(CH3)2CCH,OC2H5OC2H5CHCH(CH3)2MgBrOHOMgBrOH-(4)+CH,,①H,△,②B2H6,H2O2/OH3CH3HBr11、(1)CH3CH=CH2CH3CH2CH2Br过氧化物2NaNH22CH3CH2CH2BrHC≡CHNaC≡CNaCH3CH2CH2C≡CCH2CH2CH3液氨H2OHgSOCH3CH2CH2COCH2CH2CH2CH34,H2SO4也可通过格氏试剂增碳、水解、氧化得到。HBrMg(2)CH3CH=CH2CH3CHBrCH3CH3CH(MgBr)CH3干醚①CH3CH2CH2COCH3CH3CCCH2CH2CH3+②H3O,△CH3CH3①H2SO4CrO3/吡啶(3)CH2=CH2CH3CH2OHCH3CHO②H2O+HCCHCH=CHO2H3O2222Ag,△O△OHOHH2CCH2OOHOHMg①CH3CHOBrCH2CH2CHOBrCH2CH2CH+CH3CH(OH)CH2CH2CHO干HCl干醚②HOO3第18页FromYan_xuejing
《有机化学》课后习题参考答案(4)CrO3/吡啶稀OH-△CH3CH2OHCH3CH=CHCHO2C2H5OHCH3COOOHOC2H5CH3CHCHCH干HClOCHO25CH3OHCH3O12、A:H3CCHCHCH3B:H3CCHCCH3CH3CH3CH3CH2CH2CH3C:H3CCHCOH和H3CCHCOHCH2CH2CH3CH3O13、A:CHCH2CH3-1红外光谱1690cm为羰基吸收峰。核磁共振普δ1.2(3H)三重峰是—CH3;δ3.0(2H)四重峰是—CH2—;δ7.7(5H)多重峰为一取代苯环。OB:CH2CCH3-1红外光谱1705cm为羰基吸收峰。核磁共振普δ2.0(3H)单峰是—CH3;δ3.5(2H)单峰是—CH2—;δ7.1(5H)多重峰为一取代苯环。14、该化合物结构式为:(CH3)2COCH2CH3(反应式略)15、A:CH3COCH2CH2CH=C(CH3)2或(CH3)2C=C(CH3)CH2CH2CHOB:CH3COCH2CH2CHOO16、H3CCCH2CH(OCH3)2-1红外光谱1710cm为羰基吸收峰。核磁共振普δ2.1(3H)单峰是—CH3;δ3.2(2H)多重峰是—CH2—;δ4.7(1H)三重峰是甲氧基中的—CH3。OC2H5OC2H517、A:B:CH=CHCOCH3CH2CH2COCH3OC2H5OC2H5OHC:D:E:CH=CHCOOHCOOHCOOH(反应式略)18、(1)CH3OCH3(2)CH3C≡CCH3(3)CH3(4)H3CCH3(5)ClCH2CH2Cl(6)(CH3)3CC(CH3)3CH3-119、红外光谱1712cm为羰基吸收峰,1383、1376为C—C的吸收峰。核磁共振普δ1.00、δ1.13是—CH3;δ2.13—CH2—;δ3.52是CH。A:(CH3)2CHCOCH2CH3B:(CH3)2CHCH(OH)CH2CH3第19页FromYan_xuejing
《有机化学》课后习题参考答案C:(CH3)2C=CHCH2CH3D:CH3COCH3E:CH3CH2CHO(反应式略)第十三章羧酸及其衍生物1、(1)己酸(2)2,2,3-三甲基丁酸(3)2-氯丙酸(4)β-萘甲酸(5)3-丁烯酸(6)环己烷羧酸(7)对甲基苯甲酸甲酯(8)对苯二甲酸(9)α-萘乙酸(10)乙酸酐(11)甲基丁烯二酸酐(12)N,N-二甲基甲酰胺(13)3,5-二硝基苯甲酰氯(14)邻苯二甲酰亚胺(15)2-甲基-3-羟基丁酸(16)1-羟基环戊烷羧酸2、COOHHCCOOHCH=CHCOOH(1)(2)(3)(4)CH3(CH2)16COOHCOOHHCCOOHOOOH2CCCOOCH3NHCOCH3COOC(5)(6)O(7)(8)CH3OH2CCH2COCONH(9)NH(10)H2NCOOC2H5(11)CH2COH2CCH2CH2CONHH2NCHHCCHCHCH(12)2(13)n(14)2nH2NOOOOCOCH33、略4、(1)草酸>丙二酸>甲酸>乙酸>苯酚(2)F3CCOOH>ClCH2COOH>CH3COOH>C6H5OH>C2H5OH(3)对硝基苯甲酸>间硝基苯甲酸>苯甲酸>苯酚>环己醇5、(1)①Ag(NH3)2OH②I2+NaOH(或NaHCO3)(2)①Ag(NH3)2OH②△(3)①Br2②KMnO4(4)①FeCl3/H2O②NaHCO3(5)①AgNO3(乙酰氯有AgCl生成)②AgNO3/C2H5OH6、(1)CH3CBr(CH3)COOH(2)(CH3)2CHCH2OH(3)(CH3)2CHCOCl(4)(CH3CH(CH3)CO)2O(5)(CH3)2CHCOBr(6)(CH3)2CHCOOC2H5(7)(CH3)2CHCONH27、第20页FromYan_xuejing
《有机化学》课后习题参考答案+H3OCH3CH2CH2CHO白色结晶CH3CH2CH2CHO△CH3CH2COCH2CH3NaHSO3(饱和溶液)×2,4-二硝基苯肼CH3CH2CH2COOH×CH3CH2CH2CH2OH×+H3O黄色CH3CH2COCH2CH3△+×NaOH/HO溶于水HCHCHCHCOOH2油水分离水层322×不溶于水油层H3COCOCH38、(1)(2)CH3CH=CHCOOHH3CCOOCH3H2CCOCH2CH2(3)O(4)H2CCO(5)CO2+H2OCH2OH3CCHCHCH3+9、(1)A:H3OB:PCl3、PCl5(或SOCl2)C:NH3D:P2O5,△E:NH3,△F:NaOBr+NaOHG:H2/Pd-BaSO4COOH(2)+①C2H5MgBr②H3O,PBr3;C2H5(3)2NH3,H2NCONHCONH2OHOHOCO(4);;CNCOOHCOO10、LiAlH4PBr3(1)CH3CH2COOHCH3CH2CH2OHCH3CH2CH2Br+NaCNH3OCH3CH2CH2COOH△Cl-2HOKMnO(2)CH3CH2CH2COOHCHCHCH(OH)COOH4CH3CH2COOH22+PH△CH2CH2Br+CH2COOH(3)过氧化物NaCNH3OHBr△CH2CH2HOCH2CH2OH(4)CH3COCH2CH2CBr(CH3)2OO干HClH3CCCH2CH2CBr(CH3)2+MgCO2H3O干醚CH3COCH2CH2C(CH3)2COOH第21页FromYan_xuejing
《有机化学》课后习题参考答案CH2CH2COCH2COOH11、(1)O(2)COOCH(3)25CH2CH2COCH2COOHCOOC2H5COOHOBrOH(4)(5)(6)COCH3H3CCCOCC2H5COOHHCH3CH3CH2COCH2COOC2H5CH3CN(7)O(8)(9)(10)CH3CH2CH2COCHCOOCHNH222512、(1)HCOOCH3>CH3COOCH3>CH3COOC2H5>CH3COOCH(CH3)2>CH3COOC(CH3)3(2)O2NCO2CH3∨ClCO2CH3∨CO2CH3∨CH3OCO2CH3HCNCH3OH,H2O13、(1)HC≡CHCH2=CHCNCH2=CHCOOCH3CuCl2△HSO24+NaCNH3OCH3CH(CH3)COOH△HBr(2)CH3CH(OH)CH3CH3CH(Br)CH3+MgCO2H3OCH3CH(CH3)COOH干醚+NaCNH3OC6H5CH2COOH△Cl2(3)C6H5CH3C6H5CH2Cl光+MgCO2H3OC6H5CH2COOH干醚+(4)Cl2NaCNH3OCHCH(COOH)CH3CH2CH2COOHP△CH322+HOBrNaCNH3O(5)CH2=CH2HOCH2CH2Br△OHCH2CH2COOH+HCNH3O(6)CH3OCHOCH3OCH(OH)CNO2NCH(OH)COOH+BrHBrNaCNH3O2(7)CH2=CH2CH3CH2Br△CH3CH2COOHPCH3CH(Br)COOHC2H5OHZnCH3CH2CHOH2O+CH3CH(Br)COOC2H5CH3CH(ZnBr)COOC2H5H干醚HClH2OCH3CH(OH)CH(CH3)COOH第22页FromYan_xuejing
《有机化学》课后习题参考答案PCl5H2CH3CH2COOHCH3CH2COClCH3CH2CHOPd/BaSO4OCHOCH3CH33BrZnCHCOOCHH2O225(8)HClCOCH3CH3CCH2COOC2H5CH3CCH2COOC2H5OZnBrOH[O]Br2C2H5OHCH3CH2OHCH3COOHBrCH2COOH+PH△BrCHCOOCHZn225BrZnCH2COOC2H5干醚(9)OBrZnCHCOOCHOZnBrH2OH2OOH225CH2COOC2H5HClCH2COOH14、从氯到两个羧基的距离不同,诱导效应也不同来解释。——15、(1)CH3CO2负离子的负电荷平均分配在两个碳原子上,而CH3CH2O负离子的负电荷定域在一个氧原子上,所以较不稳定,与质子作用的倾向较大。——(2)CH3CH2CH2CO2>ClCH2CH2CO2(由于Cl的诱导作用而较稳定)———(3)ClCH2CH2CO2>CH3CH(Cl)CO2(Cl靠近—CO2中心而较稳定)——(4)FCH2CO2>F2CHCO2(含有两个氟原子)——(5)CH3CH2CH2CO2>HC≡CCH2CO2(HC≡C—吸电子诱导效应)16、10×1000×(183.5/1000)=1835克,需KOH1.835千克17、反应式略(A)的结构式为:HOOCCH2CH(C2H5)CH2COOH(B)的结构式为:HOOCCH2CH(C2H5)COOH18、(A)的结构式为:CH3COOCH=CH2(B)的结构式为:CH2=CHCOOCH3OCH2COOHCH2COOC2H5CH2COOC2H5(A)(B)(C)19、OCHCOOC2H5CHCOOH(D)CHCOOC2H5H3COCH3CH3CH320、(1)HCOOCH2CH2CH3(2)CH3CH2COOCH3(3)CH3COOCH2CH3第十四章β—二羰基化合物1、(1)2,2-二甲基丙二酸(2)2-乙基-3-丁酮酸乙酯(3)2-氧代环己烷甲酸甲酯(4)甲酰氯基乙酸(5)3-丁酮酸(乙酰乙酸)2、(1)环戊酮(2)CH3COCH2CH2CH2COOH(3)CH3CH2CH2COOH3、(1)加FeCl3/H2OCH3COCH(CH3)COOC2H5有颜色反应.第23页FromYan_xuejing
《有机化学》课后习题参考答案(2)加FeCl3/H2OCH3COCH2COOH有颜色反应.4、(1)互变异构(2)共振异构(3)互变异构5、O(1)CH3CH2CCHCOOC2H5CHOH(2)COCH(CH3)COOC2H5+C2H5OH+25CH3H3CCHCOOC2H5O(3)+C2H5OH(5)CHO+C2H5OHCOCOOC2H5CH2CO(4)H2CCHCOOC2H5+C2H5OHCH2CH2CHO6、(1);CHOCHO(2)C2H5ONa,CH3CH(Br)COOC2H5,CH3COCH2CH(CH3)COOC2H5(3)HOCH2CH2OH/干HCl,CH3COCH2C(OH)(C6H5)2(4)NaCH(COOC(C2H5OCO)2CCH2CH2COCH3HOOCCHCH2CH2COCH32H5)2,,CH2CH2HOOCCHCH2CH2CH(OH)CH3OC6H5CH2OCH2,CH3-CH=CHCOCHNaOCH3237、C6H5CH2COCH2C6H5C6H5CHCOCH2C6H5CH2CH2CH2CH2CH2CH2-CHCHCONaOCH3CHCHCOCHCHCO6565-65CCH2CH3CCHCHCCHCH33OOC6H5OC6H5C6H5CH2CH2OHCH2CH2CH3OHC6H5CHC-H2OCHCHC65CCHCH3△CHCCH3OOC6H5C6H58、丙二酸酯合成:[O]Cl2①NaCN2C3H5OHCH3CH2OH+CH2(COOC2H5)2P②HO+3H第24页FromYan_xuejing
《有机化学》课后习题参考答案NaBrNaBr(1)CH3CH2OHCH3CH2BrCH3OHCH3BrH2SO4H2SO4C2H5ONaC2H5BrCH2(COOC2H5)2NaHC(COOC2H5)2C2H5CH(COOC2H5)2①C2H5ONaH+-CO2②CHBrCH3CH2CH(CH3)COOH3H2O△+OH2H2CCH2(2)CH3CH2OHCH2=CH2AgO+△HO3NaBrMgCH3CH2OHCH3CH2BrCH3CH2MgBrH2SO4干醚NaBrCH3CH2CH2CH2OHHSOCH3CH2CH2CH2Br24CHONaCHCHCHCHBr+-COCH2(COOC2H5)2253222H2CHCHCHCHCHCOOH32222H2O△+NaBrNaCNH(3)CH3CH2OHCH3CH2BrCH3CH2CNCH3CH2COOHH2SO4H2OLiAlH+Br4H2CH3CH2CH2OHCH2=CHCH3CH3CH(Br)CH2Br△-H2O2C2H5ONaCHCH(Br)CHBr2CH2(COOC2H5)22NaCH(COOC2H5)232+H-CO2CH2CH2COOHHO△2HCCHCHCOOH321,2-二溴乙烷合成酮(3).+HBr(4)CHCHOHCH=CH2CHBrCHBr32-HO2222△22C2H5ONaCH2BrCH2Br2CH2(COOC2H5)22NaCH(COOC2H5)2CH2CH(COOC2H5)22CHONaCHBrCHBr2522CH2CH(COOC2H5)2CHOCOCOOCH+2525H-CO2HOOCCOOHC2H5OCOCOOC2H5H2O△(5)1,2-二溴乙烷合成同(4).2CHONaCHBrCHBrH+CH(COOCH)2522COOC2H5-CO22252COOHCOOC2H5H2O△9、乙酰乙酸乙酯合成:第25页FromYan_xuejing
《有机化学》课后习题参考答案CHCHOH[O]CH3CH2OHC2H5ONa32CH3COOH+CH3COOC2H5+CH3COCH2COOC2H5H△HNaBr(1)CH3CH2OHCH3CH2BrH2SO4①C2H5ONa①C2H5ONa5%NaOHCH3COCH2COOC2H5CH3CH2CH(C2H5)COCH3②C2H5Br②C2H5Br(2)CH3OHNaBrCHBr3H2SO4①C2H5ONa①C2H5ONa40%NaOHCH3COCH2COOC2H5(CH3)2CHCOOH②CH3Br②CH3Br[O]Cl2CH3CH2OH(3)CH3CH2OHCH3COOHPClCH2COOH+ClCH2COOC2H5H①C2H5ONa5%NaOHCH3COCH2COOC2H5CH3COCH2CH2COOH②ClCH2COOC2H5+BrH2(4)CH3CH2OHCH2BrCH2Br-H2O①2C2H5ONa5%NaOH2CH3COCH2COOC2H5CH3COCH2CH2CH2CH2COCH3②CH2BrCH2BrNaBrMg(5)CH3CH2OHCH3CH2BrCH3CH2MgBrHSO干醚24+CrO3/吡啶①CH3CH2MgBrHCH3OHHCHO+CH3CH2CH2OH-HO②HO23Br2HBrCH3CH=CH2BrCH2CH2CH2Br光过氧化物①2C2H5ONa5%NaOHCCHCH3COCH2COOC2H53②BrCHCHCHBr222O10、该化合物为:CH3COCH2CH2COOH反应式略。11、A:CH3CH2COOC2H5B:CH3CH2COCH(CH3)COOC2H5C:C2H5COC(C2H5)(CH3)COOC2H5D:CH3CH2COCH(CH3)CH2CH3反应式略。第十五章硝基化合物和胺1、(1)2-甲基-3-硝基戊烷(2)丙胺(3)甲异丙胺(4)N-乙基间甲苯胺(5)对氨基二苯胺(6)氢氧化三甲基异丙铵(7)N-甲基苯磺酰胺(8)氯化三甲基对溴苯铵(9)对亚硝基-N,N-二甲基苯胺(10)丙烯腈2、第26页FromYan_xuejing
《有机化学》课后习题参考答案NHCOCH3CH3CHNHHSONC2H5(1)(2)32·24(3)(4)NO2CH3CH2NH2CH2CH2CH2NH2NH2N=C=O(5)(6)CH3NC(7)(8)CH2CH2CH2NH23、(1)①Ag(NH3)2OH②CHCl3/KOH(异腈反应)③NaHCO3溶液NH2(2)CH3√溶解NaOHSO2ClNHCH√H2O不溶解3N(CH3)2×(3)CHCl3/KOH(异腈反应)(4)Br2/H2O(或用漂白粉溶液,苯胺显紫色)4、-CH3CH2CH2NH2HO√溶解CH3CH2CH2NH2(1)CHCHCHNOHCl+3222×NaOH溶于水HCHCHCHNO3222(CH3)3CNO2×H2O不溶于水-HOH2NCOOH√溶于水溶于水HNCOOH过量HCl2OHNaOHOH(2)√溶于水不溶于水H2ONH2×油(3)(CH3CH2)3N溶于水+HCH3CO(CH2)3CH3HO×√CH3CO(CH2)3CH32HO-NaHSO2△×3×HOCH3(CH2)4CH2NH2溶于水CH3(CH2)4CH2NH2CH3(CH2)4CH2OH××稀HCl不溶于水5、(1)甲胺>氨>苯胺>乙酰胺(2)苄胺>对甲苯胺>对硝基苯胺>2,4-二硝基苯胺(3)甲胺>N-甲基苯胺>苯胺>三苯胺NH2NH2NHCOCH3∨(4)∨∨(5)NHNH6、(1)CH3CH2COOH,CH3CH2COCl,CH3CH2CON(CH2CH2CH3)2,(CH3CH2CH2)3N第27页FromYan_xuejing
《有机化学》课后习题参考答案HCCH3H3CCH3CH3CH33+H3C+-(2)NHO-NCH2NHO;;CH2CH3CH3CH3CH2CH2+N(CH3)3+H2OH3COOCHCH265CH2C6H5CH2C6H5(3)NCH(COOC2H5)2;NC(COOC2H5)2;H2NC(COOC2H5)2;H2NCHCOOHOO7、[O]①SOCl2(1)(CH3)2CHCH2CH2OH(CH3)2CHCH2COOH②NH3Br2(CH3)2CHCH2CONH2(CH3)2CHCH2NH2NaOHPCl3NH3(2)(CH3)2CHCH2CH2OH(CH3)2CHCH2CH2Cl(CH3)2CHCH2CH2NH2PCl3NaCN(CH)CHCHCHCN(3)(CH3)2CHCH2CH2OH(CH3)2CHCH2CH2Cl3222H2,Ni(CH)CHCHCHCHNH322222Br2NaCNH2,Ni(4)CH2=CH22BrCH2CH2BrH2NCH2CH2CH2CH2NH2(5)CH=CHHBrCHCHBrNaCNCH3CH2CN2232Br+22NaCNHO(6)CHCH=CH3HOOCCHCH(CH)COOH32△23[O]①SOCl2Br2(7)O2NCH3-O2NNH2②NH3HO8、CH3CH3COOHCOOH混酸KMnO4Fe+HCl(1)△NO2NONH22NO2NO2NH2(2)混酸发烟H2SO4(NH4)2SHNO3△NO2NO2第28页FromYan_xuejing
《有机化学》课后习题参考答案NO2NHCOCH3(3)混酸Fe+HCl(CH3CO)2OHNO3(CH3CO)2ONHCOCH3+NH2H3OFe+HClNHNO22△CH3CH3CH3CH3CH3-(4)混酸Fe+HClH2SO4混酸O2NNO2HOO2NNO2△++NO2NH3OSO2HNH3OSO2HNH2NO2NH2混酸Fe+HCl(5)SO2NHSO3HClSO3HNO2NO2NO2混酸Zn过量NHNH(6)NaOH醇溶液CHCH3CH33混酸Fe+HCl(7)NHCH2NO2NH2CH3Cl2CH2Cl光SO3HONaOCHOCH33(8)H2SO4①NaSO3CH3IBr2②NaOH熔融FeBr3OCHOOCHBr+33①Mg干醚①H3O①NK②②PBr3OOCH2CH2NH2②NaOHH2OCH2CH2BrOCH3CH2BrNKCHNH+22CH2N(CH3)3(9)NBS①3CHBr-O3Br②NaOHH2O第29页FromYan_xuejing
《有机化学》课后习题参考答案CH3CH3COOHCOCl混酸[O]SOCl2(10)AlClCONO2NO2NO23O2NNHCOCH3混酸Fe+HClCHCOCl3NHCOCH3硝化还原乙酰化9、(3)的合成路线最合理。10、略+–11、A:CH2=CHCH2NH2B:CH3CH2CH2CH2NH2C:[CH2=CHCH2CH2N(CH3)3]ICOOHE:COOHD:CH2=CH—CH=CH2CH2CH3CH2N12、该化合物为:第十六章重氮化合物和偶氮化合物1、(1)重氮苯硫酸盐(2)对乙酰基重氮苯盐酸盐(3)4-甲基-4’-羟基偶氮苯(4)4-(N,N-二甲胺基)-4’-甲基偶氮苯(5)2,2’-二甲基氢化偶氮苯(6)二氯碳烯2、增强。因为苯基重氮盐是一种弱的亲电试剂,故当重氮基邻位或对位上连有强吸电子基硝基时,增强了重氮盐的亲电性,使偶合反应活性提高。3、重氮盐与苯胺偶合在弱酸中有利,重氮盐与酚偶合在弱碱中有利。CH3CH3CH3CH34、(1)Fe,HCl;NaNO2,HCl;;;;;OHBrCNCH3H2NH3CNNCH;3FOCH3CH3CHCHCHH322Cl(2)(3)(4)ClHCH2CH2CH2CH3第30页FromYan_xuejing
《有机化学》课后习题参考答案+N2N(CH3)25、(1)重氮组分HOS偶氮组分3OH(2)重氮组分NNN+偶氮组分2+(3)重氮组分N2偶氮组分CH3CH3CONHHO+(4)重氮组分N2偶氮组分OHNa2O3SNH2(5)++重氮组分N2N2偶氮组分SO3HNONH2ClCl2ClCl6、(1)Fe,HClFeCl3NaNO2C2H5OHCl2H2SO4ClClCH3CH3CH3CH3混酸Fe,HClFeBr3NaNO2C2H5OH(2)BrBrBrH2SO42BrBrNH2NH2NO2NH2CN混酸发烟H2SO4Fe,HClNaNO2KCN(3)HNO3NO2NH2HClCNNHCOCH3NH2OH+Br2H3O①NaNO2,H2SO4(4)②H2O,△BrBrCH3CH3CH3CH3+混酸Fe,HCl(CH3CO)2OHNO3H3O①NaNO2,H2SO4(5)NO2②C2H5OHNO2NH2NHCOCH3NH2NHCOCH3NHCOCH3BrBrBrBr(CHCO)O混酸Br+,H(6)322H3O①NaNO22SO4FeBr3②C2H5OHNO2NO2NO2第31页FromYan_xuejing
《有机化学》课后习题参考答案NH2NHCOCH3NHCOCH3NH2BrBrBrBr(CHCO)O混酸Br+(7)322H3OFeBr3NO2NO2NO2COOHCOOH+①NaNO2,HClH3OBrBrFe+HCl①NaNO2,H2SO4BrBr②KCN,CuCN②C2H5OHNO2NH2CNCOOH①NaNO2HCl+(8)H3O[O]②KCN,CuCN△CH3CH3COOHNO2NH2N(CH3)27、(1)混酸Fe,HCl2CHOH3弱酸性NNN2ClN(CH)32NaNO2,HClCH3CH3CH3混酸Fe,HClNaNO,HCl(2)2NH2NH2N2Cl弱酸性NNNO2NH2H3CNH2混酸发烟H2SO4Fe,HClHNO3NO2NH2CH3CH3CH3(3)H2SO4Na2SO3①NaOH,熔融+②HCH3SO3HOH弱碱性+CHNNCH3混酸HO①33Fe,HClHON2Cl△②NaNO2,HClNH2N2Cl混酸Fe,HClH2SO4(4)NN180℃~190℃HOS3SO3H弱碱性OHH2SO4Na2SO3①NaOH,熔融OH∧80℃+②H第32页FromYan_xuejing
《有机化学》课后习题参考答案NHCOCH3N2Cl混酸Fe,HClCHCOCl+(5)3混酸①H3OCH3COOH②NaNO2,HCl混酸Fe,HClN(CH3)2NO22CH3OH弱酸性NNO2NN2Cl(CH3)2NNO2CHCH33(6)混酸Fe,HClNaNO2,HClCH3NNH3CCH3N2ClONaOHCO2COONa(7)由苯磺化碱熔融得△,P弱碱性NNNaO3SOH由苯通过⑷法得N2ClCOONaSO3NaCH3CH3(8)H2SO4Na2SO3①NaOH,熔融OH+②HOH弱碱性NNH3CCH3CH3,HClCH3混酸Fe,HClNaNO2N2ClNO2CH38、(1)ClBr(2)NO2NH2NH2NH2NH2CH3OH9、(1),(2),(3),SO3HN(CH3)2SO3HSO3HNH2NH2NH2NN10、该化合物是:H3CN(CH3)2合成方法略。CH3H2NNNCH311、该化合物是:合成方法略。第十七章杂环化合物第33页FromYan_xuejing
《有机化学》课后习题参考答案CH3+Cl1、(1)(2)NI-(3)(4)NHCCHOOH33(5)SSO3H(6)CHO,CH2OH,OCOOHOOCOOHCH2COOH(7)(8)N(9)(10)NNNHHOHOHONa2、(1)NaOHSHO×浓HSOSO3H溶于浓硫酸224S××不溶于冷硫酸CHO(2)(3)NHC2H5OH红色CH3COOH×(无颜色反应)浸过浓盐酸的松木片×NH2红色NCHOHOCH3SO3H3、(1)S浓H2SO4S(2)×不溶于盐酸溶于浓硫酸浓HCl×不溶于冷硫酸√溶于浓盐酸NN×(3)H3CSO2Cl馏出物蒸馏NHNO2SCH3馏余物4、从杂原子对芳杂环上电子云密度影响去解释。5、(1)CH=CHCHO(2)CHOH,COOH(3),+-OO2ONNIHH3CCH3+H3OCOOH(4)(5)CH2CH2CN△H2,Pt;HCl;COCH2CH2CNH2,NiSCOOH6、(1)(2)(3)(4)SO2NSCH3SNNCH3第34页FromYan_xuejing
《有机化学》课后习题参考答案(5)NO2(6)CH3NCH3(7)H3COCH3(8)SSSH7、苄胺>氨>吡啶>苯胺>吡咯8、具有芳香性的化合物有:NNNNN,H3COCH3,N,OSH9、六元环上的两个N为吡啶型N,五元环上的两个N为吡咯型。10、(1)CHOZnO-Cr2O3-MnO2H2,PtHClOOClCH2CH2CH2CH2ClH2O,400℃~415℃NaOH,H2OHOCHCHCHCHOH2222(2)NaNH2NaNO2H2SO4HO2NNH2OHNN+(3)CH2COONO2Br2①Mg,干醚H3ONO2BrONO2HOOCONO2O-5℃~-30℃O②CO2(4)CH混酸Fe+HClHCNH332CH2OHCHOOHHOHCH2SO4CHH3CNH2H3CHCCCHOH3NCHOHCH2HNH2H2SO4H3CC6H5NO2,[O]HC3-H2ONNHOHCrO/吡啶CHO(5)CH3CH2CH2OH3CH3CH2CHOO-CHCHCHO稀HOOCH3△①Ag(NH3)2OHCHCCHOCHCCOOHO②+OHCH3CH3CHO11、原来的C5H4O2的结构是;O第十八章碳水化合物1、D-核糖,D木糖,D-阿洛糖和D-半乳糖2、D-核糖,2R,3R,4RD-阿拉伯糖,2S,3R,4R,D-木糖,2S,3S,4RD-米苏糖,2S,3S,4R3、(1)不是对映体,是差向异构体。(2)不是对映体,是差向异构体,异头物。4、(1)前者有还原性,可发生银镜反应,后者无还原性。(2)前者无还原性,后者有还原性。第35页FromYan_xuejing
《有机化学》课后习题参考答案(3)前者有还原性,后者无还原性或者前者无酸性,后者又酸性。(4)前者有还原性,后者无还原性(5)前者中性,后者酸性。5、它们与过量的苯肼能生成相同结构的脎,由此可见它们的C-3,C-4,C-5具有相同的构型。CH=NNHC6H5CH=NNHC6H5CH=NNHC6H5CH2C=NNHC6H5C=NNHC6H56、(A)(B)(C)CHCHOHCHOH2CHOHCH2CH2OHCH2OHCH2OHCH=NOHCH=NNHC6H5COOHCOOHHOHC=NNHC6H5HOHHOH7、HOH(2)HOH(3)HOHHOH(1)(4)HOHHOHHOHHOHHOHHOHHOHHOHCH2OHCH2OHCH2OHCOOHD-甘露糖肟D-甘露糖脎D-甘露糖酸D-甘露糖二酸HHHCHOCOCHCH2OCH3CH2OCH3O23OCH3OCHCOOOCH3OCH3O3CH3OHCH3OHHCH3OCH3OHCHOHCH3COOCHOCHCHOCOCH3H3HHHH(5)五乙酸-D-甘露糖酯(6)五甲基-D-甘露糖(7)四甲基-D-甘露糖OOOHOHHOHHOHHOHHOHHOH8、HOHHOHHOHHOHHOHHOHOHOHOH(A)D-太罗糖(B)D-阿卓糖(C)D-阿洛糖HCHOCHOCH2OHHOHHOHHOOH10、A为HOH或HOH9、HHOHHOHHOOHCHCH2OHCH2OHHOCOOHCOOHHCHHOHHOH2OB为或HOHOHHOHHHHOHHOHHOCHOHCOOHCOOHOHCHCHOCHOCOOHC为HOHHOHD为HOH或HOHHOHHOHCH2OHCH2OHCOOH第36页FromYan_xuejing
《有机化学》课后习题参考答案第十九章氨基酸、蛋白质和核酸NH21、(1)H2NCH2CH2CH2CH2CHCONHCH2COOHNH2CH2OH(2)HOOCCHCHCHCONHCHCONHCHCOOH22CH2CH2COOHCH(CH3)2(3)CH3CHCONHCHCONHCHCONHCHCONHCHCOOH2NH2CH2CH2CH(CH3)2NH22、CH3CHCOOHCH3CHCHCOOHC6H5CH2CHCOOHSCH2CHCOOHH2NCH2COOHNH2HONH2NH2SCH2CHCOOHNH23、(1)H3CCHCOOH溶液有明显的酸性,(2)NH溶液有明显的碱性,NHCOCH它可溶于碱而不溶于酸它可溶于酸而不溶于碱3COOCH3(3)(b)有明显酸性;(d)又明显碱性;余下(a)和(c)其中(c)可与HNO3作用有N2↑。4、(1)CH3CHCOO-(2)C6H5CH2CHCOOH+NH2NH35、(1)CH3CH2CH2CHCOOH,H2NCH2COOH,HOOCCH2CHCOOH,NH3NH2NH2(2)CH3COOH,H2NCH2COOH(3)H2NCH2CH2CH2CH2COOH第二十章元素有机化合物1、略。2、(1)对甲基苯基锂(2)二乙基膦(3)碘化四乙基鏻(4)二苯基二氯甲硅烷(5)三正丙基铝Li3、(1)(2)(CH3)4Si(3)(C2H5)3SiH(4)CH3SiH2Cl(5)(C2H5)2Si(OC2H5)2NLi4、(1)+CH3CH2CH2Br(2)CH3CH2CH3+LiOH(3)CH3CH2CH3+C2H5OLi第37页FromYan_xuejing
《有机化学》课后习题参考答案(4)CH3CHCH2CH2CH3CH3CHCH2CH2CH3(5)CH3CH2CHCCH3;CH3CH2CH2COCH3;-OLiOHO(6)(C2H5)2Hg+2(C2H5)2AlCl(7)(C2H5)3Sb+AlX3(8)CH2=P(C6H5)3CH3(9)(C6H5)3P=CHCH2CH(CH3)2;H3CCHCHCH2CH(CH3)2;H2,PtOHCH3CH2CH2CH2Li5、(1)CH3CH2CH2CH2Li+CO2CH3CH2CH2CH2COOLiCHCHCHCHC(OLi)CHCHCHCHH2OCH3CH2CH2CH2COCH2CH2CH2CH3322222223HBrMg+(2)CH3CH2OHCH3CH2BrCH3CH2MgBrH3OCHCHCH(OH)CH干醚323CrO3CH3CHO吡啶[O]CH3CH2COCH3CHFeBr3Mg66+Br2C6H5BrC6H5MgBrPCl3(C6H5)3P干醚[O]C2H5OHBr2CH3CH2OHCH3COOH+CH3COOC2H5BrCH2COOC2H5HP(C6H5)3PC2H5ONa(CH)P+CH2CH2COCH3653CHCOOC2H5CH3CH2C(CH3)=CHCOOC2H5(3)同(2)的方法合成(C6H5)3PAlCl3NBS(C6H5)3PC6H6+CH3CH2ClC6H5CH2CH3C6H5CH(Br)CH3+-C2H5ONa(H5C6)3PCC6H5CH3COCH3(H3C)2CCC6H5CH3CH3[O]SOCl2(4)C2H5OHCH3COOHCH3COClOOHNaBH4-H2OCHCH(C5H5)2Fe+CH3COClCCH3CHCH3+2H2OH△FeFeFe第38页FromYan_xuejing'
您可能关注的文档
- 最新公务员考试行测真题(答案及解析).pdf
- 最新大学毛概课后习题答案2.doc
- 最新数据结构习题及参考答案.pdf
- 最新版《毛邓》习题(同步练习)答案(独创).doc
- 有关英语语法中《句子成分及五种基本句型》问题_附有习题及答案.doc
- 有机化学(徐寿昌)课后习题答案.pdf
- 有机化学-第五版-华北师范大学等-李景宁-全册-课后习题答案(完整版).doc
- 有机化学-第五版-华北师范大学等-李景宁-全册-课后习题答案(完整版).pdf
- _第二版_徐寿昌_课后习题参考答案(全).doc
- 有机化学》(第三版)汪小兰主编课后习题答案1-3.doc
- 有机化学周莹主编课后习题参考答案.doc
- 有机化学周莹主编课后习题答案.doc
- 有机化学第6版倪沛洲主编课后习题答案汇总.doc
- 第二版_夏百根_黄乾明_主编_课后答案.doc
- 有机化学第五版下册(李景宁主编) 课后习题答案.doc
- 有机化学第五版下册(李景宁主编)-课后习题答案.doc
- 有机化学试题及答案(一).docx
- 有机化学课后习题参考答案(汪小兰第四版)(1).doc
相关文档
- 施工规范CECS140-2002给水排水工程埋地管芯缠丝预应力混凝土管和预应力钢筒混凝土管管道结构设计规程
- 施工规范CECS141-2002给水排水工程埋地钢管管道结构设计规程
- 施工规范CECS142-2002给水排水工程埋地铸铁管管道结构设计规程
- 施工规范CECS143-2002给水排水工程埋地预制混凝土圆形管管道结构设计规程
- 施工规范CECS145-2002给水排水工程埋地矩形管管道结构设计规程
- 施工规范CECS190-2005给水排水工程埋地玻璃纤维增强塑料夹砂管管道结构设计规程
- cecs 140:2002 给水排水工程埋地管芯缠丝预应力混凝土管和预应力钢筒混凝土管管道结构设计规程(含条文说明)
- cecs 141:2002 给水排水工程埋地钢管管道结构设计规程 条文说明
- cecs 140:2002 给水排水工程埋地管芯缠丝预应力混凝土管和预应力钢筒混凝土管管道结构设计规程 条文说明
- cecs 142:2002 给水排水工程埋地铸铁管管道结构设计规程 条文说明