- 1.19 MB
- 2022-04-22 11:46:26 发布
- 1、本文档共5页,可阅读全部内容。
- 2、本文档内容版权归属内容提供方,所产生的收益全部归内容提供方所有。如果您对本文有版权争议,可选择认领,认领后既往收益都归您。
- 3、本文档由用户上传,本站不保证质量和数量令人满意,可能有诸多瑕疵,付费之前,请仔细先通过免费阅读内容等途径辨别内容交易风险。如存在严重挂羊头卖狗肉之情形,可联系本站下载客服投诉处理。
- 文档侵权举报电话:19940600175。
'课后答案网您最真诚的朋友www.hackshp.cn网团队竭诚为学生服务,免费提供各门课后答案,不用积分,甚至不用注册,旨在为广大学生提供自主学习的平台!课后答案网:www.hackshp.cn视频教程网:www.efanjy.comPPT课件网:www.ppthouse.com课后答案网www.hackshp.cn
1绪论1.1在银催化剂上进行甲醇氧化为甲醛的反应:2CHOH++O→2HCHO+2HO3222CHOH++3O→2CO+4HO3222进入反应器的原料气中,甲醇:空气:水蒸气=2:4:1.3(摩尔比),反应后甲醇的转化率达72%,甲醛的收率为69.2%。试计算(1)(1)反应的选择性;(2)(2)反应器出口气体的组成。解:(1)由(1.7)式得反应的选择性为:Y0.629S===0.961196.11%0.960.961196.11%1196.11%=X0.7200.720.7200(2)进入反应器的原料气中,甲醇:空气:水蒸气=2:4:1.3(摩尔比),当进入反应器的总原料量为100mol时,则反应器的进料组成为khdaw.com组分摩尔分率yi0摩尔数ni0(mol)CH3OH2/(2+4+1.3)=0.274027.40空气4/(2+4+1.3)=0.547954.79水1.3/(2+4+1.3)=0.178117.81总计1.000100.0设甲醇的转化率为X课后答案网A,甲醛的收率为YP,根据(1.3)和(1.5)式可得反应器出口甲醇、甲醛和二氧化碳的摩尔数nA、nP和nc分别为:nA=nA0AA00(1-(1-X(1-XXA)=7.672)=7.672molmolnP=n==nnA0AA00YP=18.96==18.96=18.96mol18.96molmmololnC=n==nnA0AA00www.hackshp.cn(X((XXA-Y--YYP)=0.7672))=0.7672)=0.7672=0.7672molmol结合上述反应的化学计量式,水(nW)、氧气(nO)和氮气(nN)的摩尔数分别为:nW=nW0+n++nnP+2n++2n2nC=38.30==38.30=38.30mol38.30molmmololnO=n==nnO0OO00-1/-1/2n-1/2n2nP-3/2n-3/2nC=0.8788=0.8788molmolmmololnN=n==nnN0NN00=43.28==43.28=43.28mol43.28molmmolol所以,反应器出口气体组成为:组分摩尔数(mol)摩尔分率%CH3OH7.6726.983HCHO18.9617.26H2O38.334.87CO20.76720.6983O20.87880.7999N243.2839.391.1.2工业上采用铜锌铝催化剂由一氧化碳和氢合成甲醇,其主副反应如下:CO+2H⇔CHOHCHCHOHOH232CO+4H⇔(CH)O(CH(CH)O)O+HO2322CO++3H⇔CH+HO242khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
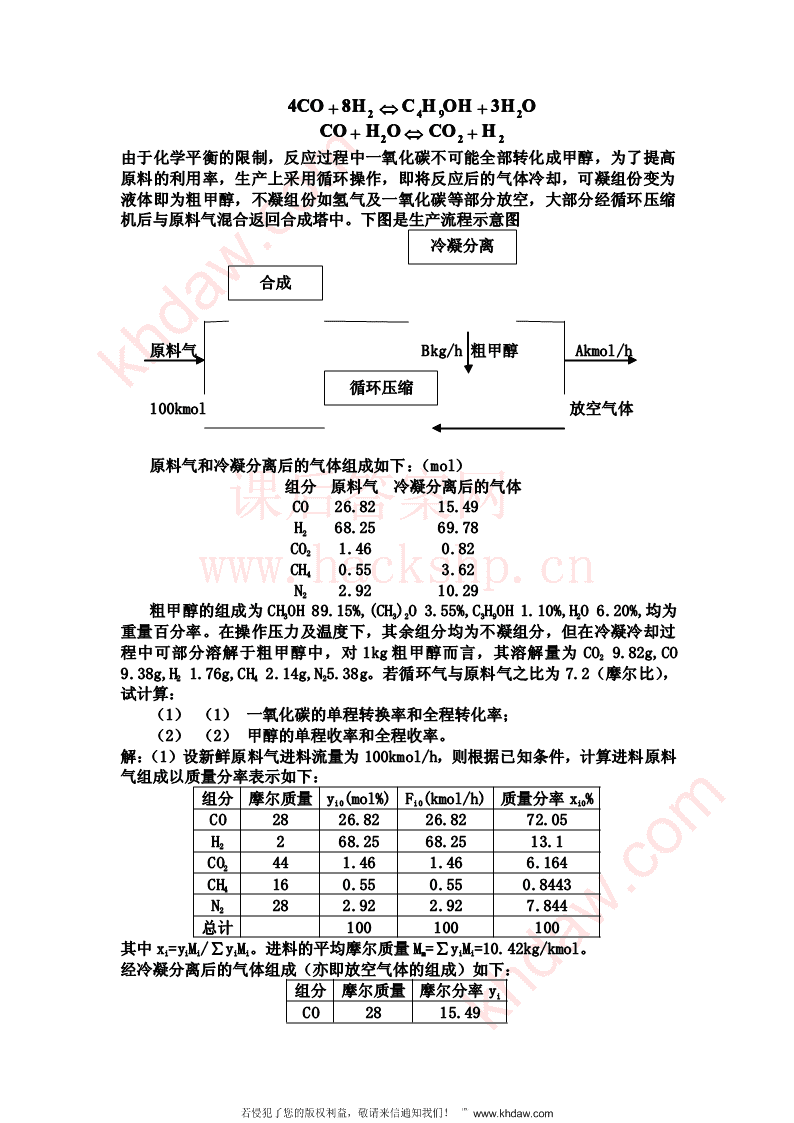
4CO++8H⇔CHOHCCHOHHOH+3HO2492CO++HO⇔CO+H222由于化学平衡的限制,反应过程中一氧化碳不可能全部转化成甲醇,为了提高原料的利用率,生产上采用循环操作,即将反应后的气体冷却,可凝组份变为液体即为粗甲醇,不凝组份如氢气及一氧化碳等部分放空,大部分经循环压缩机后与原料气混合返回合成塔中。下图是生产流程示意图冷凝分离合成原料气Bkg/h粗甲醇Akmol/hkhdaw.com循环压缩100kmol放空气体原料气和冷凝分离后的气体组成如下:(mol)组分原料气冷凝分离后的气体课后答案网CO26.8215.49H268.2569.78CO21.460.82www.hackshp.cnCH40.553.62N22.9210.29粗甲醇的组成为CH3OH89.15%,(CH3)2O3.55%,C3H9OH1.10%,H2O6.20%,均为重量百分率。在操作压力及温度下,其余组分均为不凝组分,但在冷凝冷却过程中可部分溶解于粗甲醇中,对1kg粗甲醇而言,其溶解量为CO29.82g,CO9.38g,H21.76g,CH42.14g,N25.38g。若循环气与原料气之比为7.2(摩尔比),试计算:(1)(1)一氧化碳的单程转换率和全程转化率;(2)(2)甲醇的单程收率和全程收率。解:(1)设新鲜原料气进料流量为100kmol/h,则根据已知条件,计算进料原料气组成以质量分率表示如下:组分摩尔质量yi0(mol%)Fi0(kmol/h)质量分率xi0%CO2826.8226.8272.05H2268.2568.2513.1CO2441.461.466.164CH4160.550.550.8443N2282.922.927.844总计100100100其中xi=yiMi/∑yiMi。进料的平均摩尔质量Mm=∑yiMi=10.42kg/kmol。经冷凝分离后的气体组成(亦即放空气体的组成)如下:组分摩尔质量摩尔分率yiCO2815.49khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
H2269.78CO2440.82CH4163.62N22810.29总计100其中冷凝分离后气体平均分子量为M’m=∑yiMi=9.554又设放空气体流量为Akmol/h,粗甲醇的流量为Bkg/h。对整个系统的N2作衡算得:5.38B/28×1000+0.1029A=2.92(A)对整个系统就所有物料作衡算得:100×10.42=B+9.554A(B)联立(A)、(B)两个方程,解之得khdaw.comA=26.91kmol/hB=785.2kg/h反应后产物中CO摩尔流量为FCO=0.1549A+9.38B/(28×1000)将求得的A、B值代入得FCO=4.431kmol/h故CO的全程转化率为课后答案网F−F2626.8226.824.435.824.435−4.435CO,0COX===83.48%83.483.48%8%COF26.8226.826.822COCO,0COCO,0,0由已知循环气与新鲜气之摩尔比,可得反应器出口处的CO摩尔流量为F’CO,0COCO,0,0=100www.hackshp.cn×0.2682+0.2682+7.27.2×100×0.1549=138.40.1549=138.4kmol/hkmkkmol/hmol/hol/h所以CO的单程转化率为F−F26.822626.824.435.824.435−4.435"CO,0COX===16.18%16.116.18%8%COF138.4138.138.44CO,0COCO,0,0产物粗甲醇所溶解的CO2、CO、H2、CH4和N2总量D为(9.829.381.762.145.38)B++++D==0.02848Bkmol/h0.020.02848Bkmol/h848Bkmol/h100010100000粗甲醇中甲醇的量为(B-D)X甲/Mm=(785.2-0.02848B)×0.8915/32=21.25kmol/h所以,甲醇的全程收率为Y总=21.25/26.82=79.24%甲醇的单程收率为Y单=21.25/138.4=15.36%22反应动力学基础反应动力学基础反应动力学基础反应动力学基础2.1在一体积为4L的恒容反应器中进行A的水解反应,反应前A的含量为12.23%(重量),混合物的密度为1g/mL,反应物A的分子量为88。在等温常压下不断取样分析,测的组分A的浓度随时间变化的数据如下:反应时间(h)1.02.03.04.05.06.07.08.09.0CA(mol/L)0.90.610.420.280.170.120.080.0450.03试求反应时间为3.5h的A的水解速率。khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
解:利用反应时间与组分A的浓度变化数据,作出CA~t的关系曲线,用镜面法求得t=3.5h时该点的切线,即为水解速率。切线的斜率为−0.76α==−0.125mollh/.6.16.1由(2.6)式可知反应物的水解速率为−dCAr==0.125mollh/.Adt2.2在一管式反应器中常压300℃等温下进行甲烷化反应:CO+3H→CH+HO242催化剂体积为10ml,原料气中CO的含量为3%,其余为N2,H2气体,改变进口原料气流量Qkhdaw.com0进行实验,测得出口CO的转化率为:Q0(ml/min)83.367.650.038.529.422.2X(%)203040506070试求当进口原料气体流量为50ml/min时CO的转化速率。解:是一个流动反应器,其反应速率式可用(2.7)式来表示−dFAr=AdV课后答案网RF=F(1−X)=QC(1−X)AA0A0A0AdF=−QCdXAwww.hackshp.cn0A0A故反应速率可表示为:dXdXr=QCA=CAA0A0A0dVdV((/Q)RR0用XA~VR/Q0作图,过VR/Q0=0.20min的点作切线,即得该条件下的dXA/d(VR/Q0)值α。VR/Q0min0.120.1480.200.260.340.45XA%20.030.040.050.060.070.00.650.04−α==1.791.1.79790.340.0.3434故CO的转化速率为C==PA0=0.10130.030.1010.10130.0330.03×=6.38106.3816.3810×0−4moll/A0RT−38.314108.3148.31410×10×573r=CdXA=6.38106.3816.3810×0−4×1.791.1410=×−3moll/.minAA0dV((/Q)R02.3已知在Fe-Mg催化剂上水煤气变换反应的正反应动力学方程为:0.85−0.400.4.4r=kyykmolkgh/⋅wCOCO2式中yCO和yCO2为一氧化碳及二氧化碳的瞬间摩尔分率,0.1MPa压力及700K时反2应速率常数kW等于0.0535kmol/kg.h。如催化剂的比表面积为30m/g,堆密度为31.13g/cm,试计算:khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
(1)(1)以反应体积为基准的速率常数kV。(2)(2)以反应相界面积为基准的速率常数kg。(3)(3)以分压表示反应物系组成时的速率常数kg。(4)(4)以摩尔浓度表示反应物系组成时的速率常数kC。解:利用(2.10)式及(2.28)式可求得问题的解。注意题中所给比表面23的单位换算成m/m。33(1)k=ρk=1.13101.1.13101310××0.0535=60.46kmolmh/.vbwρbρb−62(2)k=k=k=1.78101.71.7810810×kmolmh/.gaw3wvρb×3010303010×10(3)(3(3))k=()1nk=(1)0.450.0.4545×0.0535=0.1508kmolpPw0.10130.1010.10133kghMPa..(....(()0.450.0.4545−33(4)k==(RT)nk=(8.31108.3118.3110×0×700)0.4500.0.45.4545×0.0535=0.333(m))0.45(kmol)khdaw.comcPw0.10.1kmolkgh.2.4在等温下进行液相反应A+B→C+D,在该条件下的反应速率方程为:1.50.5r=0.8CCmoll/⋅minmmininAAB若将A和B的初始浓度均为3mol/l的原料混合进行反应,求反应4min时A的转化率。解:由题中条件知是个等容反应过程,且A和B的初始浓度均相等,即为课后答案网1.5mol/l,故可把反应速率式简化,得1.50.5222r=0.8CC=0.80.0.88C=0.8C(1−X)AABAA0A由(2.6)式可知www.hackshp.cn−dCdC⎡(1(1−X)⎤dXA⎢⎣⎢⎣⎢⎣A0A⎥⎦⎥⎦⎥⎦Ar===−==−−=CAA0dtdtdt代入速率方程式CdXA=0.8C2((11−X)2A0A0Adt化简整理得dXA=0.80.8CdtA0(1(1−X)A积分得X0.80.8Ct=AA01−XA解得XA=82.76%。2.5氨合成塔入口的气体组成为3.5%NH3,20.8%N2,62.6%H2,7.08%Ar,7.08%A,7.08%Arr及5.89CH5.89C5.89CHH4。该塔是在30MPa压力下操作。已知催化剂床层中某处的温度为490℃,反应气体中氨含量为10%(mol),试计算该处的反应速率。在Fe催化剂上氨合成反应速率式为:1.51.5ppr=kpH2−kNH3kmolm/3⋅h1N2p2p1.51.5NH3H2khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
������4逆反应的活化能E=17.581017.5817.5810×10Jmol/。450℃时30.532k=2277mMPa()/m⋅hkk/=K2,且12P,490℃时,Kp可按下式计算:−4−72logKp=2047.8/202047.8/47.8/T−2.4943logT−1.25610×T+1.856410×T+3.2063注:m为标准立方米。解:题中给出450℃时的k2值,而反应是在490℃下,故首先要求出490℃时的k2值。利用(2.27)试,求出频率因子A:��k=Aexp(eexp(xp(−E)2RT������4A=kexp(−E)=2277/e−−17.5810/8.31472317.5817.5810/8.314723×10/8.314723×=1.14510×162RT������4khdaw.comk"=Aexp(−E)1.14510))1.14510=1.14510×16e−−17.5810/8.3147231717.5810/8.314723.5810/8.314723××=1.05510×4mMPa3()/0.5mh3.3.2RT490℃的Kp值由题给公式计算出−4−72logKp=2047.8/7632.4943log7631.256102047.8/2047.8/7632.4943log7631.256107632.4943log7631.25610−−××7631.8567631.856410+4104104×10×763++3.206=−1.2524−2Kp=5.592105.59210×课后答案网×求k1值:2k12"K=k=Kkp"1p2kwww.hackshp.cn2−22222243−1.511.5.53k=(5.59210)(5.59(5.59210)210)××1.05510×=33mMPa()/mh.1求各组分的分压值:13N+H↔NH22322khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
νip−pXi0A0AνAp=i1+yδXA0AAνiy−yXi0A0AνAy=,p=yPiii1+yδXA0AA131-(+)22y=20.87%,δ==-2A0A12νRy−yXR0A0AνAy=R1+yδXkhdaw.comA0AA1.0035−*.02087*XA1−2.010=1+.02087*(-2)*XA45.914X=5.6课后答案网AX=.01416Ay−yXA0A0Ay=A1+yδXwww.hackshp.cnA0AA.02087(1.0-1416)=1+.02087*(-2)*0.1416=.019043y−yXH0A0A1y=H1+yδXA0AA.06263-*.02087*0.1416==.057161+.02087*(-2)*0.1416p=yPii各组分的分率及分压值为NH310%pNH3=3MPaN219.06%pN2=5.718MPaH257.18%pH2=17.15MPaAr+CH413.79%pAr+CH4=4.137MPa反应速率为:1.51.5pp1.51.54r=kpH2−kNH3=33.05.71833.0533.05.718×.718×17.15117.157.15−1.05510××31N2p2p1.51.5317.15117.157.151.51.1.51.55NH3H23333=4.023104.0234.02310×10m/mcath.(179.6kmolmcath/.)2.6下面是两个反应的T-X图,图中AB是平衡曲线,NP是最佳温度曲线,khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
AM是等温线,HB是等转化率线。根据下面两图回答:(1)(1)是可逆反应还是不可逆反应?(2)(2)是放热反应还是吸热反应?(3)(3)在等温线上,A,D,O,E,M点中哪一点速率最大,哪一点速率最小?khdaw.com(4)(4)在等转化率线上,H,C,R,O,F及B点中,哪一点速率最大,哪一点速率最小?(5)(5)在C,R两点中,谁的速率大?(6)(6)根据图中所给的十点中,判断哪一点速率最大?解:图2.1图2.2(1)可逆反应课后答案网可逆反应(2)放热反应吸热反应(3)M点速率最大,A点速率最小M点速率最大,A点速率最小(4)O点速率最大,B点速率最小www.hackshp.cnH点速率最大,B点速率最小(5)R点速率大于C点速率C点速率大于R点速率(6)M点速率最大根据等速线的走向来判断H,M点的速率大小。2.7在进行一氧化碳变换反应动力学研究中,采用B106催化剂进行试验,4测得正反应活化能为9.629109.6299.62910×10Jmol/,如果不考虑逆反应,试问反应温度是550℃时的速率比反应温度是400℃时的速率大多少倍?解:从题中可知,反应条件除了温度不同外,其它条件都相同,而温度的影响表现在反应速率常数k上,故可用反应速率常数之比来描述反应速率之比。Aexp(eexp(xp(−E)E1196962909962901629029011rkRTRT(−T)(−)550555500==550555500=550=e400550=e8.3146738238.3148.314673823673823=23(倍)rkA−E400400404000exp(exeexp(xp(p()RT4004002.8常压下,在钒催化剂上进行SO2氧化反应,原料气组成为7%O2及82%N2。试计算转化率为80%时的最佳温度。二氧化硫在钒催化剂上氧化的正反应活化能4为9.211109.2119.21110×10Jmol/,化学计量数等于2,反应式为:1SO+O↔SO2232其平衡常数与温度的关系为:logKp=4905.5/Te−4.6455khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
4该反应的热效应−−Hr=9.629109.6299.62910×10Jmol/。解:(1)求出转化率为80%时各组分的分压:以100mol为基准xSO2O2SO3N2∑07.011.0082.0100.00.807(1-0.80)=1.411-5.6×0.5=8.25.6082.097.2−3p=Py=0.100.1010.10131.4/97.21.4610(131.4/97.21.4610(31.4/97.21.4610(×=×MPa)SO2SO2−3p=Py=0.10138.2/97.20.1010.10138.2/97.238.2/97.2×=8.5510(×MPa)O2O2−3p=Py=0.10135.6/97.20.1010.10135.6/97.235.6/97.2×=5.8410(×MPa)SO3SO3−2p=Py=0.101382/97.20.1010.101382/97.2382/97.2×=8.5510(×MPa)N2N2khdaw.com(2)求与上述组成对应的平衡常数KP值:p−3K=SO3=5.845.8415.8410×100=43.26443.263.26P0.0.500.5.550.5pSOpo1.46101.4611.4610×0−3⎛8.5510×−3⎞22⎜⎟⎝⎠(3)求平衡温度Telog课后答案网Kp=4905.5/Te−4.64554905.54905.4905.55Te==780.97780.980.9K6.28266.282.282(4)利用(2.31)式求逆反应活化能E值��www.hackshp.cn��4E=E−∆Hr=9.211109.2119.21110×104−−9.6299.629.62910910×10=1.40310×5Jmol/ν2r(5)利用(2.31)式求最佳温度TOPT=Te������=780.97780.980.9=739.004739.0739.00404KOP11+��RTe��e��lnE���E��E���1+8.314780.98.3148.314780.9×780.9ln14.03(14.039.211)10(14.0(14.039.211)1039.211)1049.21199.211.211E−EE−×2.9在一恒容反应器中进行下列液相反应:3AB+→RrR=1.61.1.66CkmolmA/⋅h232A→DrD=8.28.8.22CkmolmA/⋅h式中rR,rD分别表示产物R及D的生成速率。反应用的原料为A与B的混合物,3其中A的浓度为2kmol/m,试计算A的转化率达到95%时所需的反应时间。解:反应物A的消耗速率应为两反应速率之和,即2R=r+2r=1.6C+16.4116.46.4C=1.6C(110.25+C)ARDAAAA利用(2.6)式dC−−A=1.6C(110.25+C)AAdt积分之khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
⎡(1(1−X)+1⎤110.25110.250.25A10.25110.250.25C1.6t=∫CA−(−)dC=−ln(1lln(1n(1−X)ln+⎢A0⎥CA0C10.25C+1AA⎢11⎥AA⎢+⎥10.25110.250.25C⎣A0⎦t=0.6463/1.60.6460.6463/1.63/1.6=0.4038h2.10在催化剂上进行三甲基苯的氢解反应:CHCH()+H→CHCH()+CH6333264324CHCH()++H→CHCH+CH643226534反应器进口原料气组成为66.67%H2,33.33%三甲基苯。在0.1Mpa及523K下等温反应,当反应器出口三甲基苯的转化率为80%时,其混合气体的氢含量为20%,试求:(1)(1)此时反应器出口的气体组成。(2)(2)若这两个反应的动力学方程分别为:khdaw.com0.53r=630066300300CCkmolm/⋅hAAB0.53r=340033400400CCkmolm/⋅hECB则出口处二甲基苯的生成速率是多少?解:以100mol为计算基准,设X为三甲基苯的转化率,Y为生成的甲苯摩尔数。课后答案网(1)(1)用物料衡算求出口气体组成:组分名称X=0时X=0.8时三甲基苯(A)33.3333.33(1-X)www.hackshp.cn氢(B)66.6766.67-33.33X-Y二甲基苯(C)033.33X-Y甲烷(D)033.33X+Y甲基苯(E)0Y∑100.0100.0由题给条件可知,混合气中氢的含量为20%,所以有:66.67-33.33X-Y=20解得Y=66.67-33.33×0.8-20=20.01kmol(甲苯量)生成的二甲基苯量:33.33×0.8-20.01=6.654kmol生成的甲烷量:33.33×0.8+20.01=46.67kmol剩余的三甲基苯量:33.33×(1-0.8)=6.666kmol氢气含量为:20kmol故出口尾气组成为:三甲基苯6.666%,氢气20%,二甲基苯6.654%,甲烷46.67%,甲基苯20.01%。(2)(2)由题给条件可知,三甲基苯的出口浓度为:pA00.10.0.100.10.3333×.33333333−33C===7.669107.6697.66910×10kmolm/A0RT−38.314108.3148.31410×10×523−3−33C=C(10.8)7.66910(10.8)1.53410(10.8(10.8)7.66910(10.8)1.53410−)7.66910(10.8)1.53410=×−=×kmolm/AA0khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
C=0.2000.0.20.2020×1.534101.5341.53410×10−3=4.610×−3kmolm/3B0.066660.0660.0666666C=0.066540.0660.0665454×1.534101.5341.53410×10−3=1.53210×−3kmolm/3C0.066660.0660.0666666C=0.46670.4660.46677×1.534101.5341.53410×10−3=1.07410×−3kmolm/3D0.066660.0660.0666666C=0.20010.2000.20011×1.534101.5341.53410×10−3=4.60310×−3kmolm/3E0.066660.0660.06666660.50.5R=r−r=6300CC−340033400400CCCAEABCB−3−30.5−3−30.5330.50.5=63001.534106300163001.53410×.53410××(4.610)×−34001.53210×××(4.610)×3==0.65550.35330.30220.6550.65550.35330.302250.35330.3022−=kmolmh/.khdaw.com2.11在210℃等温下进行亚硝酸乙脂的气相分解反应:11CHNO→NO+CHCHO+CHOH25232522该反应为一级不可逆反应,反应速率常数与温度的关系为144−1k=1.3910exp(1.89710/)(1.3911.3910exp(1.89710/)(×0exp(1.89710/)(−×Ts),若反应是在恒容下进行,系统的起始总压为0.1013MPa,采用的是纯亚硝酸乙脂,试计算亚硝酸乙脂分解率为课后答案网80%时,亚硝酸乙脂的分解速率及乙醇的生成速率。若采用恒压反应,乙醇的生成速率又是多少?解:(1)恒容过程,其反应式可表示为:www.hackshp.cn11A→→B+C+D22反应速率式表示为:r==kC=kC(1−X)AAA0A设为理想气体,反应物A的初始浓度为:C=PyA0=0.0.1010.101310133=2.523102.5232.52310×10−2moll/A0RT−38.314108.3148.31410×10×48314r=kC(1−X)1.3910exp(18973/))1.)1.3910exp(18973/)=3910exp(18973/)×−T×C(1−X)AA0AA0A1411414−2−6=1.3910exp(18973/483)2.523101.3911.3910exp(18973/483)2.52310×0exp(18973/483)2.52310−×××(100.8)6.1121(100.8)6.11210=×00molls/.亚硝酸乙脂的分解速率为:−6R=r=6.112106.1126.11210×10molls/.AA乙醇的生成速率为:1−6R=r=3.056103.0563.05610×10molls/.DA2(2)恒压过程,由于反应前后摩尔数有变化,是个变容过程,由(2.49)式可求得总摩尔数的变化。10.50.51++−δA=∑υυt/A==11由于反应物是纯A,故有:yA0=1。由(2.52)式可求得组分的瞬间浓度:khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
−2CA0(1(1−XA)2.5232.52310(10.8)×10(10.8)−−2C===2.803102.8032.80310×10moll/A1+δyX1110.81110.1110.8++××+××+××8AA0A14−2−6r=kC=1.3910exp(18973/483)2.803101.3911.3910exp(18973/483)2.80310×0exp(18973/483)2.80310−××=3.39510×molls/.AA乙醇的生成速率为:1−6R=r=1.698101.6981.69810×10molls/.DA22.12甲烷与水蒸气在镍催化剂及750℃等温下的转化反应为:CH++2HO→CO+4H4222原料气中甲烷与水蒸气的摩尔比为1:4,若这个反应对各反应物均为一级,已知k=2l/mol.s,试求:(1)(1)反应在恒容下进行,系统的初始总压为0.1013MPa,当反应器出口khdaw.com的CH4转化率为80%时,CO2和H2的生成速率是多少?(2)(2)反应在恒压下进行,其他条件如(1),CO2的生成速率又是多少?解:(1)由题意可将反应速率表示为:A+2B→C+4Dr=kCCCAB对于恒容过程,则有C=C(1(1−X)A课后答案网A0AC=C−2CXBB0A0APA00.10130.20.1010.10130.230.2×−3C====2.382102.3822.38210×10moll/A0www.hackshp.cnRT8.314108.3148.31410×10−3×1023−3−3C=4C=×==×42.3821042.342.38210×8210×=9.52810×moll/B0A0当XA0=0.8时−3−4C=C(1−X)=22.2.38210.3821038210××0.24.76410=×moll/AA0A−3−3−3C=C−2CX=9.528109.529.52810810×−×−−×22.38210×××0.85.71710=×moll/BB0A0A−4−3−6R=r=kCC=×24.7641024.7624.76410410××5.71710×=5.44710×molls/.CCAB−6−5R=4r=×45.4471045.445.447104710×=2.17910×molls/.DC(2)对于恒压过程,是个变容反应过程,由(2.49)式可求得总摩尔数的变化1412+−−δA=∑υυt/A==21反应物A的原始分率:1y==0.20.2A014+由(2.52)式可求得转化率为80%时的浓度:−3CA0(1(1−XA)2.3822.38210(10.8)×10(10.8)−−4C===3.609103.6093.60910×10moll/A1+δyX120.20.8120.2120.20.8++×+×+××0.8AA0A−3−3CB0−2CXA0A9.52899.52810.52810×10−×22.38210××0.8−5C===4.331104.3314.33110×10moll/B1+δyX120.20.8120.2120.20.8+×++×+××0.8AA0Akhdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
−4−5−6r=kCC=×23.6091023.6023.60910910××4.33110×=3.12610×molls/.AAB−6R=r=3.126103.1263.12610×10molls/.CC2.13在473K等温及常压下进行气相反应:r=1.2Cmoll/⋅min(1)A→3RRAr=0.5Cmoll/⋅min(2)A→2SSAr=2.1Cmoll/⋅min(3)A→TTA式中CA为反应物A的浓度(mol/l),原料中A和惰性气体各为一半(体积比),试求当A的转化率达85%时,其转化速率是多少?δ解:方法(1),先求出总摩尔变化数A。首先将产物的生成速率变为对应的反应物的转化速率:1khdaw.comrAR=rR=0.40.4CA31r=r=0.250.0.2525CASSA2r=r=2.12.1CATTA总反应速率为:课后答案网R=r+r+r=2.75CAARASATA以一摩尔反应物A为基准,总摩尔变化数为:0.40.252.1δA=www.hackshp.cn×+3×+2−=10.38210.10.3823822.752.7522.75.752.75初始浓度为:C=Py0A0=0.0.1010.10130.510130.530.5×=1.288101.2881.28810×10−2moll/A0−3RT8.314108.3148.31410×10×473则有−2C=CA0(1−XA)=1.1.288101.1.2881028810××0.15=1.6625101.66251.662510×10−3moll/A1+δyX10.50.380.8510.50.3810.50.380.85++××0.85AA0A−3−3R=2.75C=2.751.6625102.751.2.751.662510×662510×=4.57210×moll/.minAA方法(2),可将CA表示为:C(1−X)A0AC=A1+yA0∑δAjXAj331−1δ==2AR121221−1δAS==11δ=0ATkhdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
0.40.0.20.25255∑δAjXAj=×2×0.8510.850.851++×1××0.85=0.32452.752.7522.75.75−21.288101.2881.28810×10×0.15−3C==1.6623101.6621.662310310×moll/A10.32450.510.3210.32450.5+450.5×−3−3R=2.75C=2.751.66231022.751.662310.751.662310××=4.57110×moll/.minAA方法(3),利用物料衡算可分别求出反应物A生成R及S的瞬间选择性SR,SS,因而可求出产物R及S的收率yR,yS,求得A转化率为85%时的分率:y(1−y−y−y)y=A0RST=0.064530.06453A12112+2yy+yyA0RA0S−2−3C=2Cy=1.288101.1.2881028810××0.0645321.6623100.0.0645321.6623100645321.662310×=××==×moll/AAOA−3−3R=2.75C=2.751.6623102.751.2.751.662310×662310×=4.57110×moll/.minkhdaw.comAA其中:0.4C0.4AS==R(0.40.252.1)(0.40(0.40.252.1)+.252.1)+C2.75A0.25C0.2500.25.25AS==S(0.40.252.1)(0.40(0.40.252.1)+.252.1)+C2.75课后答案网A0.40.4y==×0.85=0.123600.1236.1236R2.752.2.75750.250.0.25www.hackshp.cn25y==×0.85=0.0772700.07727.07727S2.752.2.75752.14在Pt催化剂上进行异丙苯分解反应:CHCHCH()⇔CH+CH65326636以A,B及R分别表示异丙苯,苯及丙烯,反应步骤如下:(1)A+σ⇔Aσ(2)Aσ⇔Bσ+R(3)Bσ⇔B+σ若表面反应为速率控制步骤,试推导异丙苯分解的速率方程。解:根据速率控制步骤及定态近似原理,除表面反应外,其它两步达到平衡,描述如下:pθA+σ⇔AσK=AVθ=KpθAAAAVθA��Aσ⇔Bσ+Rr=kθ−kpθAARBpθBσ⇔B+σK=BVθ=KpθBBBBVθB以表面反应速率方程来代表整个反应的速率方程:��r=kθ−kpθAARBkhdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
θ+θ+θ=1由于ABVθθ,将AB代入上式得:Kpθ+Kpθ+θ=1AAVBBVV整理得:1θ=V1+Kp+KpAABBθθθ,,将ABV代入速率方程中��kKpkpKpkp(−pp)/KAARBBABRPr=−=A1+Kp+Kp1+Kp+Kp1+Kp+KpAABBAABBAABB其中���k=kKK=kK/kKkhdaw.comAPAB2.15在银催化剂上进行乙烯氧化反应:2CH+O→2CHO242242()(A+B)→2()2(2()R)化作2其反应步骤可表示如下:课后答案网(1)A+σ⇔AσB+2σ⇔2Bσ(2)2www.hackshp.cn(3)Aσ+Bσ⇔Rσ+σ(4)Rσ⇔R+σ若是第三步是速率控制步骤,试推导其动力学方程。解:根据速率控制步骤及定态近似原理,除表面反应步骤外,其余近似达到平衡,写出相应的覆盖率表达式:(1)A+σ⇔Aσθ=KpθAAAV(2)((2)2)B+2σ⇔2Bσθ=Kpθ2BBBV(4)Rσ⇔R+σθ=KpθRRRV整个反应的速率方程以表面反应的速率方程来表示:��r=kθθ−kθθAABRV根据总覆盖率为1的原则,则有:θ+θ+θ+θ=1ABRV或Kpθ+Kpθ+Kpθ+θ=1AAVBBVRRVV整理得:1θ=VKp+Kp+KpAABBRRθθθθ,,,将ABRV代入反应速率方程,得:khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
�2�2kp(ApB−pR/Kr=kKpKpθ−kpKθ=AAABBVRRV2(1(1++Kp+Kp+Kp)AABBRR其中��k=kKKK=kkK/ABR2.16设有反应A→B+D,其反应步骤表示如下:(1)A+σ⇔Aσ(2)Aσ→Bσ+D(3)Bσ⇔B+σ若(1)速率控制步骤,试推导其动力学方程。解:先写出各步的速率式:(1)(1)A+σ⇔Aσr=kpθ−kθkhdaw.com1aAAVdAA(2)(2)Aσ→Bσ+Dr=kθ2SA(3)(3)Bσ⇔B+σr=kθ−kpθ3dBBaBAV由于(1)是速率控制步骤,第(2)步是不可逆反应,其反应速率应等于(1)的吸附速率,故有:kpθ−kθ=kθaAAVdAASA整理得:课后答案网kpθθ=aAAVAk+kwww.hackshp.cnSdA根据定态近似原则dθB=kθ−kθ+kpθ=0SAdBBaAAVdtkkpSaAAkθ=kθ+kpθ=(+kp)θdBBSAaAAVaBBVk+kSaAkkpSaAAθ=(+kp)θ/kBaBBVdBk+kSaAθ+θ+θ=1因为ABVθθ,将AB代入上式,化简后得:1θ=VkpaAA1++KpBBk+kSdAθθ,最后将AV代入吸附速率式,即为该反应的动力学方程式。kpaAAkp−kaAAdAk+kkkpSdASaAAr==1kpaAAkS+kdA+kpaAA+(kS+kdA)KpBB1++KpBBk+kSdAkhdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
2.17一氧化碳变换反应:COA()+HOB()→COC()(())+HD()222在较低温度下,其动力学方程可表示为:kppr=AB1+Kp+KpAACC试拟定该反应的合适的反应步骤。解:根据题意,假设反应步骤如下:(1)A+σ⇔Aσ(2)(2)Aσ+B→Cσ+D(3)(3)Cσ⇔C+σ并假设第二步是控制步骤,其速率方程就代表了整个反应的速率方程:r=kθpkhdaw.comSAB其余的两步可认为达到平衡,则有:θ=KpθAAAVθ=KpθCCCVθ+θ+θ=1由于ABV,有:θ=课后答案网1V1+Kp+KpAACCθθ,将AV代入速率式,得:www.hackshp.cnkKppkppr=SAAB=AB1+Kp+Kp1+Kp+KpAACCAACCk=kK式中SA。故上述假定与题意符合。但上述假定的反应步骤不是唯一的。2.18利用习题2.1的数据,试用积分法和微分法求其动力学方程。解:先用积分法求其动力学方程。设为一级不可逆反应,其速率表达式为:dCr=−A=kCAAdt积分得:Ckt=ln(lnln((A0)CACln(lnln((A0)C用A~t作图。t(h)0123456789Cln(lnln((A0)00.440.831.251.602.102.452.863.433.84CA213987283-1作图得一直线,其斜率为0.425h,故假设一级反应是合理的,其动力学方程可khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
表示为:dCr=−=−A=0.425Cmollh/.AAdt用微分法求解动力学方程首先用CA~t曲线,在曲线上取时间为0,1,2,……9h所对应点的切线,为了准确可采用镜面法,求得各切线的斜率即为对应的dCA/dt之值,然后再以dCA/dt~CA作图,所求的dCA/dt值如下表所示:t(h)0123456789CA(mol/l)1.40.90.610.400.280.170.10.080.040.030025dCA/dt(mol/l0.70.40.290.190.140.090.00.030.020.01.h)0551756355-1设为一级不可逆反应,用dCkhdaw.comA/dt~CA作图得一直线,其斜率为0.5h,其动力学方程可表示为:dCr=−=−A=0.5Cmollh/.AAdt或将速率方程直线化,即两边取对数,得:dCln(−−A)ln=k+nlnCA课后答案网dt可简化为y=b+ax形式,利用多元回归,可求得反应级数n=1.004≈1,反应速率常数值为k=0.4996。还可用一数学公式来拟合Cwww.hackshp.cnA~t曲线,然后再将该数学公式对t求导,求得dCA/dt之值,此法会比作图法来的准确。2.19在Ni催化剂上进行甲烷化反应:CO++3H⇔CH+HO242由实验测得200℃时甲烷的生成速率RCH4及CO和H2的分压pCO,pH2的关系如下:pCO(MPa)0.100.180.4080.721.05pH2(MPa)0.10130.10130.10130.10130.1013RCH4mol7.33107.3317.3310×0−31.32101.3211.3210×0−23.00103.0013.0010×0−25.28105.2815.2810×0−27.70107.7017.7010×0−2()g⋅minmin若该反应的动力学方程可用幂函数表示,试用最小二乘法求一氧化碳的反应级数及正反应速率常数。解:由题意可写出速率方程式:aβr=kp"pCH4COH2但由于氢的分压保持为0.1013MPa的恒定值,反应速率式可简化为:ar=kpCH4COβk=kp"式中H2。将速率式直线化:khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
lnrCH4=lnk+alnllnnpCO或y=+baxy=lnr,b=ln,lnln,kx,=+lnp式中CH4CO,由残差平方和最小而导出最小二乘法的系数计算式:∑∑∑∑∑x∑y−m∑xya=22⎡⎣⎡⎣⎡⎣⎡∑x⎤⎦⎤⎦⎤⎦⎤−−m∑x1b=⎡⎣⎡⎣⎡⎣∑ya−∑x⎤⎦⎤⎦⎤⎦m2序号yxxxy1-4.916-2.3035.30211.322-4.328-1.7152.9417.421khdaw.com3-3.507-0.8970.8043.1444-2.941-0.3290.1080.9665-2.564-0.0490.002-0.125∑-18.26-5.2939.15722.73将累加值代入a,b系数式中,得:(18.26)(5.293)522.73(18.2(18.26)(5.293)522.73−6)(5.293)522.73×−−×a==0.95710.9570.9571≈1课后答案网2(5.293)(5.29(5.293)−−3)−×59.1571b=[−18.260.957(5.293)18.2618.260.957(5.293)−0.957(5.293)×−]=−2.6395www.hackshp.cn−2k=7.144107.1447.14410×10−2R==rCH=7.144107.1447.14410×10pkmolg/.minCH44CO2.20在铂催化剂上,乙烯深度氧化的动力学方程可表示为:kppr=AB2(1(1+Kp)BB式中pA,pB分别表示乙烯及氧的分压。在473K等温下的实验数据如下:334号pA×10MPapB×10MPar×10mol/g.min18.9903.2300.672214.223.0001.07238.8604.0800.59848.3202.0300.71354.3700.8900.61067.7501.7400.83477.7501.8200.82886.1701.7300.65696.1301.7300.694106.9801.5600.791112.8701.0600.418khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
试求该温度下的反应速率常数k和吸附平衡常数KB。解:首先将动力学方程式直线化:kpppp1Kr=AB⇒AB=+Bp2B(1(1+Kp)rkkBB或y=b+ax。其中pp1KABBy=,,b=,a=,x=pBrkk32534序号yx×10x×10xy×10r〞×10δ,%10.6573.3201.0432.1230.6780.9020.6313.0000.9001.8921.1265.030.7784.0801.6653.1720.5685.0khdaw.com40.4872.0200.4120.9880.82916.350.2530.8900.0790.2250.5824.660.4021.7400.3030.7000.8330.170.4131.8200.3310.7510.8161.480.4031.7300.2990.6980.6651.490.3911.7300.2990.6760.6614.810课后答案网0.3711.5600.2430.5790.7870.5110.2701.0600.1120.2860.36911.7∑5.05622.875.66812.09δ=4.7www.hackshp.cn−2−22.2872.2.2871028710×10×5.056111.20910−××a==169.11169.169.12−4−52.287×10−115.68810××1−2b=⎡5.056169.12.287105.0565.056169.12.28710−169.12.28710××⎤=0.10811⎣⎦1k==8585.73885.735.73.732b3K=ak=169.1×85.73=1.56610×B85.73885.735.73ppr=AB23(11.5661011.5611.56610+610×pB)平均偏差δ=4.7%,结果是令人满意的。33釜式反应器釜式反应器釜式反应器釜式反应器3.1在等温间歇反应器中进行乙酸乙酯皂化反应:CHCOOCH+NaOH→CHCOONa+CHOH325325该反应对乙酸乙酯及氢氧化钠均为一级。反应开始时乙酸乙酯及氢氧化钠的浓度均为0.02mol/l,反应速率常数等于5.6l/mol.min。要求最终转化率达到95%。试问:3(1)(1)当反应器的反应体积为1m时,需要多长的反应时间?khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
3,(2)(2)若反应器的反应体积为2m,所需的反应时间又是多少?XAfdXXAfdX1XAAAt=C=C=×A0∫0(−R)A0∫0kC22((11−X)2kC1−XAA0A0AA0A10.95=×=169.6min(2.83)169.6169.6min(2.83)min(2.83)h解:(1)5.60.0210.955.60.5.60.0210.95×0210.95−(2)因为间歇反应器的反应时间与反应器的大小无关,所以反应时间仍为2.83h。3.2拟在等温间歇反应器中进行氯乙醇的皂化反应:CHClCHOH+NaHCO→CHOHCHOH+NaCl+CO223222以生产乙二醇,产量为20㎏/h,使用15%(重量)的NaHCO3水溶液及30%(重量)的氯乙醇水溶液作原料,反应器装料中氯乙醇和碳酸氢钠的摩尔比为1:1,混合液的比重为1.02。该反应对氯乙醇和碳酸氢钠均为一级,在反应温度下反khdaw.com应速率常数等于5.2l/mol.h,要求转化率达到95%。(1)(1)若辅助时间为0.5h,试计算反应器的有效体积;(2)(2)若装填系数取0.75,试计算反应器的实际体积。解:氯乙醇,碳酸氢钠,和乙二醇的分子量分别为80.5,84和62kg/kmol,每小时产乙二醇:20/62=0.3226课后答案网kmol/h0.326680.5×=91.11kgh/每小时需氯乙醇:0.9530%0.9530.9530%×0%0.326684×www.hackshp.cn=190.2kgh/每小时需碳酸氢钠:0.9515%0.9510.9515%×5%91.11190.291.1191.11190.2+190.2Q==275.8/275.8275.8/lh/0原料体积流量:1.021.1.02020.326610000.3260.3266100061000×C==1.231moll/A0氯乙醇初始浓度:0.95275.80.9520.95275.8×75.8反应时间:XAfdX1XAfdX10.90.955t=CA=A=×=2.96822.968.968hA0∫∫20kCCkC0(1−X)5.21.23110.955.5.21.23110.9521.23110.95×−ABA0AV=Qt(+t")=275.8(2.968275.8(275.8(2.968×2.968+0.5)=956.5l反应体积:r0V956.5rV===127512127575lf0.750.0.7575(2)(2)反应器的实际体积:3.3丙酸钠与盐酸的反应:CHCOONa+HCl⇔CHCOOH+NaCl2525为二级可逆反应(对丙酸钠和盐酸均为一级),在实验室中用间歇反应器于50℃等温下进行该反应的实验。反应开始时两反应物的摩尔比为1,为了确定反应进行的程度,在不同的反应时间下取出10ml反应液用0.515N的NaOH溶液滴定,以确定未反应盐酸浓度。不同反应时间下,NaOH溶液用量如下表所示:khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
时间,min010203050∝NaOH用量,ml52.232.123.518.914.410.5现拟用与实验室反应条件相同的间歇反应器生产丙酸,产量为500kg/h,且丙酸钠的转化率要达到平衡转化率的90%。试计算反应器的反应体积。假定(1)原料装入以及加热至反应温度(50℃)所需的时间为20min,且在加热过程中不进行反应;(2)卸料及清洗时间为10min;(3)反应过程中反应物密度恒定。解:用A,B,R,S分别表示反应方程式中的四种物质,利用当量关系可求出任一时刻盐酸的浓度(也就是丙酸钠的浓度,因为其计量比和投量比均为1:1)为:0.515C=C=×Vmoll/ABNaOH10于是可求出A的平衡转化率:C−C52.510.552.5152.510.5−0.5A0AeX===0.80.8khdaw.comAeC52.55252.5.5A0X=X×90%0.890%0.7290%0.90%0.890%0.72=890%0.72×=AAe0.51500.515.515C=C(1−X)=×52.5(10.72)0.051514.75252.5(10.72)0.051514.7.5(10.72)0.051514.7×−=×moll/AA0A10现以丙酸浓度对时间作图:课后答案网由上图,当CA=0.0515×14.7mol/l时,所对应的反应时间为48min。由于在同样条件下,间歇反应器的反应时间与反应器的大小无关,所以该生产规模反应www.hackshp.cn器的反应时间也是48min。丙酸的产量为:500kg/h=112.6mol/min。所需丙酸钠的量为:112.6/0.72=156.4mol/min。Q=F/C=156.4/(0.0515156.4/(0.156.4/(0.05150515+52.5)=57.84/minl原料处理量为:0A0A0V=Qt(+t)=57.84(18×+2010)+=4512l反应器体积:r00实际反应体积:4512/0.85640=l3.4在间歇反应器中,在绝热条件下进行液相反应:AB+→R141100011100010003r=1.110exp(1.1101.110exp(×exp(−)CCkmolmh/.AAB其反应速率方程为:T式中组分A及B的浓度CA及CB以kmol/m3为单位,温度T的单位为K。该反应的热效应等于-4000kJ/kmol。反应开始时溶液不含R,组分A和B的浓度均等于330.04kmol/m,反应混合物的平均热容按4.102kJ/m.K计算。反应开始时反应混合物的温度为50℃。(1)(1)试计算A的转化率达85%时所需的反应时间及此时的反应温度。(2)(2)如果要求全部反应物都转化为产物R,是否可能?为什么?解:(1)khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
C(−∆H)0.04×−−[(4000)((4000)4000)]A0rT=T+(X−X)323))323=323+X=32339.01332339.012339.01+X0AA0AAC4.10244.102.102F0XAfdXAXAfdXAt=C=CA0∫0(−R)A0∫0kCCAABdXA=C=9191.32991.321.32.32hA0∫1100011100010001414221.110exp(1.1101.110exp(×exp(−)C(1−X)A0A32339.013233932339.01+.01XA(由数值积分得出)T=32339.010.85+×=356.2K(2)若A全部转化为R,即XA=1.0,则由上面的积分式知,t→∝,这显然是不可能的。khdaw.com3.5在间歇反应器中进行液相反应:AB+→Cr=kCCA1ABC+B→Dr=kCCD2CB3A的初始浓度为0.1kmol/m,C,D的初始浓度为零,B过量,反应时间为t1时,333CA=0.055kmol/m,CC=0.038kmol/m,而反应时间为t2时,CA=0.01kmol/m,课后答案网3CC=0.042kmol/m,试求:(1)(1)k2/k1;(2)(2)产物C的最大浓度;(3)(3)对应C的最大浓度时A的转化率。www.hackshp.cn解:(1)因为B过量,所以:""""r=kCr,=kCr,=r−r=kC−kCA1AD2CCAD1A2C恒容时:−dCA=kC"1Adt(A)−dCC=kC"−kC"1A2Cdt(B)(B)式除以(A)式得:"dCkCC2C−=−1"dCkCA1A解此微分方程得:"⎡k2⎤"C⎢⎛C⎞k1C⎥A0AAC=⎜⎟−Ck"⎢CC⎥1−2⎢⎝A0⎠A0⎥k"⎣⎦1(C)将t1,CA,CC及t2,CA,CC数据代入(C)式化简得:xx0.420.55×−0.380.1×=0.420.55×−0.380.1×解之得:khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
"kk22x==0.520.52500.525.5255="kk11(2)先求出最大转化率:1k2−1X=−1(k1)k1=0.74250.0.74257425A,max,m,maxaxk2(3)产物C的最大收率:1⎡k2⎤YC,max,m,maxax=⎢(1−XAk1)−(1−XA⎥=)0.49050.40.4905905k1−2⎣⎦k1产物C的最大浓度:khdaw.comC=CY=0.10.49050.04910.10.0.10.49050.0491×49050.0491=kmolm/3C,maxA0C,max3.6在等温间歇反应器中进行液相反应A⇔←⎯→k1A⎯⎯→k3A123初始的反应物料中不含A2和A3,A1的浓度为2mol/l,在反应温度下-1-1-1k1=4.0min,k课后答案网2=3.6min,k3=1.5min。试求:(1)(1)反应时间为1.0min时,反应物系的组成。(2)(2)反应时间无限延长时,反应物系的组成。www.hackshp.cnA⎯⎯→k1A⇔←⎯→k3A(3)(3)将上述反应改为123反应时间无限延长时,反应物系的组成。解:根据题中给的两种反应情况,可分别列出微分方程,然后进行求解。但仔细分析这两种情况,其实质是下述反应的特例:A←⎯→k1A←⎯→k2A123(A)"k=0A⇔A→A当2时,(A)式变为123(B)"k=0A→A⇔A当1时,(A)式变为123(C)""k=0,0,k=0A→A→A当12时,(A)式变为123(D)其中式(D)即为书讲的一级不可逆连串反应。可见只要得到(A)式的解,则可容易化简得到(B),(C)及(D)式的解。对于(A)式,可列出如下微分方程组:dC1"−=kC−kC1111dt(1)dC2""=kC+kC−kC−kC11231222dt(2)dC3"=kC−kC2223dt(3)由题意知初始条件为:khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
C(0)=C,C(0)((0)0)=C(0)=011023(4)联立求解此微分方程组可得:⎧⎪kk""kk"⎡(α+ke")αt(β+ke")βt⎤⎫⎪211122C=C⎨+⎢−⎥⎬110⎪αβα−β⎣αα(+k)ββ(+k)⎦⎪⎩11⎭(5)""αt"βt⎧⎪kkk⎡(α+ke)(β+ke)⎤⎫⎪112122C=C⎨+⎢−⎥⎬210⎪αβα−β⎣αβ⎦⎪⎩⎭(6)⎪⎧kkkk⎡eαteβt⎤⎫⎪1212C=C⎨+⎢−⎥⎬310⎪αβα−β⎣αβ⎦⎪⎩⎭(7)αβ,式中,由如下式确定:"""khdaw.comαβ=kk12+kk12+kk212211(8)""α+β=−(k+k+k+k)1122(9)现在可用上述结果对本题进行计算:−1"−1−1"k=4.0mi4.0m4.0min,in,n,k=3.6min,k=1.5min,k=0,t=1min(1)1122由(5)~(9)式得课后答案网C=0.55920.5590.55922moll/A1C=0.50980.5090.50988moll/A2www.hackshp.cnC=0.931moll/A3(2)当t→∝时,由(5)~(9)式得C=C=0C→2.0moll/A1A2A3"−1k=0k=4.0min,4.0mi4.0min,n,(3)此时为1的情况,当t→∝时,由1−1"−1k=1.5m1.5mi1.5min,in,n,k=3.6min22得:C=0A1C=1.412moll/A2C=0.588moll/A33.7拟设计一反应装置等温进行下列液相反应:2A+2B→Rr=kCCR1AB22AB+→Sr=kCCS2AB目的产物为R,B的价格远较A贵且不易回收,试问:(1)(1)如何选择原料配比?(2)(2)若采用多段全混流反应器串联,何种加料方式最好?(3)(3)若用半间歇反应器,加料方式又如何?khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
2νrkCC1S=AR=1AB=νRkCC2+2kCC22kCBA1AB2AB2A1+kC解:(1)1B由上式知,欲使S增加,需使CA低,CB高,但由于B的价格高且不易回收,故应按主反应的计量比投料为好。(2)保证CA低,CB高,故可用下图所示的多釜串联方式:(3)用半间歇反应器,若欲使CA低,CB高,可以将B一次先加入反应器,然后滴加A.33.8在一个体积为300l的反应器中86℃等温下将浓度为3.2kmol/m的过氧化氢异丙苯溶液分解:CHCCH()COH→CHCOCH+CHOH65323365-1生产苯酚和丙酮。该反应为一级反应,反应温度下反应速率常数等于0.08skhdaw.com,最终转化率达98.9%,试计算苯酚的产量。(1)(1)如果这个反应器是间歇操作反应器,并设辅助操作时间为15min;(2)(2)如果是全混流反应器;(3)(3)试比较上二问的计算结果;(4)(4)若过氧化氢异丙苯浓度增加一倍,其他条件不变,结果怎样?解:(1)课后答案网XAfdXAXAfdXA11t=C=C=lnllnnA0∫A0∫0(−R)0kC(1((11−X)k1−Xwww.hackshp.cnAA0A0AA11=ln=56.3756.56.3737s=0.94min0.810.9891010.989−.989V=Qt(+t)=Q(0.9415)300(0.(0.9415)3009415)300+=lr0000Q=300/15.9418.82/min300/15.9418.300/15.9418.82/min=82/minl0C=CX=3.20.9893.20.3.20.989×989=3.165moll/苯酚浓度苯酚A0AQC=18.823.16559.5618.8218.823.16559.56×3.16559.56=mol/min=335.9kgh/苯酚产量0苯酚(2)全混流反应器QCXQX0A0Af0AfV==rkC(1−X)k(1−X)A0AfAfVk(1(1−X)3000.08(10.989)3000.3000.08(10.989)×08(10.989)−rAfQ===0.2669/0.2660.2669/9/ls=16.02/minl0X0.98900.989.989AfQC=16.023.20.98950.6916.0216.023.20.98950.69×3.20.98950.69×=mol/min=285.9kgh/苯酚产量0苯酚(3)说明全混釜的产量小于间歇釜的产量,这是由于全混釜中反应物浓度低,反应速度慢的原因。(4)由于该反应为一级反应,由上述计算可知,无论是间歇反应器或全混流反应器,其原料处理量不变,但由于CAB增加一倍,故C苯酚也增加一倍,故上述两个反应器中苯酚的产量均增加一倍。khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
3.9在间歇反应器中等温进行下列液相反应:3AB+→Rr=1.611.6.6Ckmolmh/.RA232A→Dr=8.2Ckmolmh/.DArD及rR分别为产物D及R的生成速率。反应用的原料为A及B的混合液,其中A3的浓度等于2kmol/m。(1)(1)计算A的转化率达95%时所需的反应时间;(2)(2)A的转化率为95%时,R的收率是多少?(3)(3)若反应温度不变,要求D的收率达70%,能否办到?(4)(4)改用全混反应器操作,反应温度与原料组成均不改变,保持空时与(1)的反应时间相同,A的转化率是否可达到95%?(5)(5)在全混反应器中操作时,A的转化率如仍要求达到95%,其它条件khdaw.com不变,R的收率是多少?(6)(6)若采用半间歇操作,B先放入反应器内,开始反应时A按(1)计33算的时间均速加入反应器内。假如B的量为1m,A为0.4m,试计算A加完时,组分A所能达到的转化率及R的收率。解:(1)第二章2.9题已求出t=0.396h=24.23min(2)dCdC−dC1.61.6C1S=(课后答案网R)/(−A)=R=A=R2dtdtdC1.6C+16.41616.4.4C110.25+CAAAA3C=C(1−X)2(10.95)0.1)2()2(10.95)0.1=10.95)0.1−=kmolm/Awww.hackshp.cnA0ACA0.10.1112C=−SdC=−=lnln(11ln(110.25(110.25+0.25C)R∫CA0RA∫2110.25110.2110.25+5C10.25A0.10.1A3=0.23050.2300.23055kmolm/C0.23050.2300.23055RY===0.11530.1150.11533RC2A0(3)若转化率仍为0.95,且温度为常数,则D的瞬时选择性为:28.2×C32.8(1−X)S=A=AD1.616.41.6161.616.4+.4C34.432.8−XAAD的收率:XAf0.9532.832.8(32.8(1(11−X)Y=SdX=AdX=0.83480.8340.83488≻0.7D∫DA∫A0034.432.834.4334.432.8−2.8XA这说明能使D的收率达到70%(4)对全混流反应器,若使τ=t=0.3958h,则有C−C0.39580.3950.39588=A0A21.6C+16.41616.4.4CAA解之得:CA=0.4433khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
C−CX=A0A=0.77840.7780.77844≺0.95AC所以:A0这说明在这种情况下转化率达不到95%。(5)(5)对全混流反应器,若X=0.95,则R的收率为:XAf0.950.0.9595Y=SX===0.46910.4690.46911RAf110.25110.2110.25+5C(1−X)110.252(10.95)+×−A0Af(6)依题意知半间歇式反应器属于连续加料而间歇出料的情况。为了求分组A的转化率及R的收率,需要求出A及R的浓度随时间的变化关系,现列出如下的微分方程组:dVC(A)+(1(1.6.6C+16.4116.46.4CV2)=QCAA0A0对A:dt(1)对R:khdaw.comdVC()R−1.61.1.66CV=0Adt(2)V=V+Qt00(3)3V=1m03在反应时间(t=0.4038h,为方便起见取t≈0.4h)内将0.4m课后答案网的A均速加入反0.40.43Q==1m/h0应器内,故0.0.40.44www.hackshp.cn3采用间歇釜操作时,原料为A与B的混合物,A的浓度为2kmol/m.现采用半间33V=1mV,=0.4m歇釜操作,且BA,故可算出原料A的浓度为:(10.4)2(10.4(10.4)2+)2×3C=kmolm/A00.40.4由于:dVC()dVdCAA=C+VAdtdtdtdVC()dVdCRR=C+VRdtdtdtdV=Q0dt代入(1),(2)式则得如下一阶非线性微分方程组:dCA=7−CA−1.6C−16.41616.4.4C2AAdt1+t(4)dCCR=1.6C−RAdt1+t(5)初始条件:t=0,CA=0,CR=0可用龙格---库塔法进行数值求解。取步长△t=0.02h,直至求至t=0.4h即可。khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
用t=0.4h时的CA和CR可以进行A的转化率和R的收率计算:N−NCV−CVX=A0A=A0AAANCVA0A0A3式中VA为所加入的A的体积,且VA=0.4m;CA0为所加入的A的浓度,且33CA0=7kmol/m;V为反应结束时物系的体积,V=1.4m。CVY=RRCV同理可以计算出R的收率:A0A3.10在两个全混流反应器串联的系统中等温进行液相反应:232A→Br=686688Ckmolmh/.AA3B→Rr=14Ckmolmh/.RB33加料中组分A的浓度为0.2kmol/m,流量为4m/h,要求A的最终转化率为90%,试问:khdaw.com(1)(1)总反应体积的最小值是多少?(2)(2)此时目的产物B的收率是多少?(3)(3)如优化目标函数改为B的收率最大,最终转化率为多少?此时总反应体积最小值是多少?解:(1)V=V课后答案网+V=QCX0A0A1+QC0A0(XA2−XA1)rr1r22222kC(1−X)kC(1−X)11A0A12A0A21+X1A1−=0www.hackshp.cn21−X(1((11−X)对上式求dVr/dXA1=0可得:A1A23100(100(11−X)=+1X将X=0.9代入上式,则A1A1A2解之得XA1=0.741所以总反应体积的最小值为4⎡0.7400.741.74110.90.741−⎤3V=V+V=⎢+⎥=3.2494.6767.9253.2493.2494.6767.925+4.6767.925=mrr1r222680.2(10.741)680.2680.2(10.741)×⎣(10.741)−(10.9)−⎦(2)2R=343344C−14CBABC=C(1−X)0.0518)0.)0.0518=0518A1A0Af2R=34C−14C=0.091231400.0912314.0912314−CB1A1B1B1VC−Cτ=r1=B1B01QR0B13.24933.249.249C=B140.09123140.090.091231412314−C即B13解得CB1=0.005992kmol/mkhdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
4.6764.674.6766C−CC−0.00599200.005992.005992τ==B2B1=B2222434C−14C34C(1−X)−14C同理A2B2A0A2B23解得CB2=0.00126kmol/mC20.0012620.0020.00126×126Y=2B2==1.26%1.261.26%%BC0.20.2B的收率:A0(3)目标函数改为B的收率,这时的计算步骤如下:对于第i个釜,组分A,B的衡算方程分别为:C−CAi−1Ai=τ2i68C对A:AiC−CBiBi−1=τ2i34C−14C对B:khdaw.comAiBi当i=1时,C−CA0A1=τ2168CA1(1)CB1=τ21343344C−14CA1课后答案网B1(2)当i=2时,C−CA1A2=τ2www.hackshp.cn268CA2(3)CB2=τ22343344C−14CA2B2(4)由(1)式解出CA1代入(2)式可解出CB1;由(1)式解出CA1代入(3)式可解出CA2;将CB1及CA2代入(4)式可解出CB2,其为τ1,τ2的函数,即C=fC(,,,,,ττ,)B2A012(5)式中CA0为常数。由题意,欲使CB2最大,则需对上述二元函数求极值:∂C∂CB2=0,0,B2=0∂τ∂τ12V=Q(τ+τ)联立上述两个方程可以求出τ1及τ2。题中已给出Q0,故由r012可求出CB2最大时反应器系统的总体积。将τ1,τ2代入(5)式即可求出B的最高浓度,从而可进一步求出YBmaX.将τ1,τ2代入CA2,则由XA2=(CA0-CA2)/CA0可求出最终转化率。33.11在反应体积为490cm的CSTR中进行氨与甲醛生成乌洛托品的反应:4NH+6HCHO→(CH)N+6HO32642式中(A)--NH3,(B)—HCHO,反应速率方程为:2r=kCCmolls/.AABkhdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
3k=1.4210exp(3090/)1.4211.4210exp(3090/)×0exp(3090/)−T式中。氨水和甲醛水溶液的浓度分别为31.06mol/l和6.23mol/l,各自以1.50cm/s的流量进入反应器,反应温度可取为36℃,假设该系统密度恒定,试求氨的转化率XA及反应器出口物料中氨和甲醛的浓度CA及CB。解:4.0644.4.06.0606C==2.0322.03.03moll/A026.326.6.3232C==3.1633.16.16moll/B023Q=×21.5=3.033.0.0cm/s0C=C(1(1−X)AA0Akhdaw.com6C=C−CX=3.161.52.033.1613.161.52.03−.52.03×XBB0A0AA43k=1.4210exp(3090/309)0.064471.4211.4210exp(3090/309)0.06447×0exp(3090/309)0.06447−=QCXQCX0A0Af0A0AfV==r26kCCABkC(1−X)(C−CX)2课后答案网A0AB0A0A43XAf490490=20.06447(10.0640.06447(147(1−X)(3.161.52.03−×X)即得:www.hackshp.cnAfAf32X−3.075X+3.162X−1.0770=整理得:AfAfAf解得:XAf=0.821反应器出口A,B得浓度分别为:C=C(1−X)2.03(10.821)0.3634)2.)2.03(10.821)0.3634=03(10.821)0.3634×−=moll/AA0A6C=C−CX=3.161.52.030.8210.66023.1613.161.52.030.8210.6602−.52.030.8210.6602××=moll/BB0A0A43.12在一多釜串联系统,2.2kg/h的乙醇和1.8kg.h的醋酸进行可逆反应。各3个反应器的体积均为0.01m,反应温度为100℃,酯化反应的速率常数为4.76-4-4×10l/mol.min,逆反应(酯的水解)的速率常数为1.63×10l/mol.min。反3应混合物的密度为864kg/m,欲使醋酸的转化率达60%,求此串联系统釜的数目。解:等体积的多釜串联系统C−Cτ=Ai−1Ai()(A)kCC−kCC1AB2CDA,B,C,D分别代表乙酸,乙酸乙酯和水。由计量关系得:C=C−(C−C)BB0A0AC=C=C−CCDA0Akhdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
从已知条件计算出:1.88641.8861.8864×4C==6.4866.48.48moll/A04.06044.060.060×2.28642.2862.2864×4C==10.33moll/B04.04644.046.046×0.01864600.0180.0186460×6460×τ==129.6min4.04.0将上述数据代入(A)式,化简后得到:2C=0.040560.0400.0405656C+1.5113C−0.887()BAi−1AiAi20.040.0400.0405605656C+1.5113C−0.887=C=6.48若i=1,则(B)式变为:A1A1A0C=4.364mollX/,=0.3260.0.326326解之得:A1A1khdaw.com0.040.0400.0405605656C2+1.5113C−0.887=C=4.364若i=2,则(B)式变为:A2A2A1C=3.20mollX/,=0.5060.0.506506解之得:A2A120.040.0400.0405605656C+1.5113C−0.887=C=3.2若i=3,则(B)式变为:A3A3A2C=2.53mollX/,=0.6090.0.609609解之得:课后答案网A3A1即:三釜串联能满足要求。3.13以硫酸为催化剂,由醋酸和丁醇反应可制得醋酸丁酯。仓库里闲置着两台33反应釜,一台的反应体积为3mwww.hackshp.cn,另一台则为1m。现拟将它们用来生产醋酸丁酯,3初步决定采用等温连续操作,原料中醋酸的0.浓度为0.15kmol/m,丁酯则大量3过剩,该反应对醋酸为2级,在反应温度下反应速率常数等于1.2m/h.kmol,要求醋酸的最终转化率不小于50%,这两台反应釜可视为全混反应器,你认为采用怎样的串联方式醋酸丁酯的产量最大?为什么?试计算你所选用的方案得到的醋酸丁酯产量。如果进行的反应是一级反应,这两台反应器的串联方式又应如何?解:因为反应级数大于1,所以联立方式应当是小釜在前,大釜在后才能使醋酸丁酯产量最大。现进行计算:VCX1r1A0A1==22QkC(1((1(1−X)Q0A0A10VC(X−X)3r2A00A2A1==22QkC(1(1−X)Q0A0A20二式联立化简后得到:(将XA2=0.5代入)2300.52.0.52.75.52.75−75X+2.5X−X=0A1A1A1解之得:XA1=0.22322kCA0(1(1−XA1)1.20.15(10.223)1.20.1.20.15(10.223)×15(10.223)−×13Q=V==0.48730.4870.48733m/h0r1X0.22300.223.223A1QCX=0.48730.150.50.48730.150.0.48730.150.5××5=36.55molh/醋酸丁酯产量=0A0A2khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
如果进行的是一级反应,可进行如下计算:(1)(1)小反应器在前,大反应器在后:VCX1r1A0A1==QkC(1((1(1−X)Q0A0A10VC(X−X)3r2A00A2A1==QkC(1(1−X)Q0A0A20联立二式,且将XA2=0.5代入,化简后得到:2X−3X+00.50.50=A1A1解得:XA1=0.1771Q=k(1−XA1)=1.2(10.1771)1.1.2(10.1771)2(10.1771)−=5.576m3/h0X0.17710.1770.17711所以有:A1醋酸丁酯产量=khdaw.comQC0A0XA2=5.5760.150.55.5760.150.5.5760.150.5××5=0.4182kmolh/(2)大反应器在前,小反应器在后:VCx3r1A0A1==QkC(1(1−x)Q0A0A10VC(x−x)1r2A00A2A1=课后答案网=QkC(1(1−x)Q0A0A20解得XA1=0.3924Qwww.hackshp.cn=3(1k−XA1)=31.2(10.3924)331.2(10.3924)×1.2(10.3924)−=5.575m3/h0X0.39240.3920.39244所以有:A1产量同前。说明对此一级反应,连接方式没有影响。3.14等温下进行1.5级液相不可逆反应:A→BC+。反应速率常数等于1.51.5335m/kmol.h,A的浓度为2kmol/m的溶液进入反应装置的流量为1.5m/h,试分别计算下列情况下A的转化率达95%时所需的反应体积:(1)全混流反应器;(2)两个等体积的全混流反应器串联;(3)保证总反应体积最小的前提下,两个全混流反应器串联。解:(1)全混流反应器V=QCX0A0A=1.520.951.5201.520.95××.95=18.02118.028.02m3r1.1.551.511.5.51.5kC(1−X)52××(10.95)−A0A(2)(2)两个等体积全混流反应器串联QCX0A0A1V=r11.511.5.51.5kC(1(1−X)A0A1QC(X−X)0A0A2A1V=r21.511.5.51.5kC(1(1−X)A0A2V=V由于r1r2,所以由上二式得:khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
X(X−X)A1=A2A11.511.5.51.5(1−X)(1−X)A1A2将XA2=0.95代入上式,化简后得到XA1=0.8245,所以:V=QCX00A0A1=2.372.37922.379.3799m3r11.511.5.51.5kC(1(1−X)A0A13V=2V=4.7584.754.7588m串联系统总体积为:rr13V=18.03118.038.03m(3)(3)此时的情况同(1),即r333.15原料以0.5m/min的流量连续通入反应体积为20m的全混流反应器,进行液相反应:A→Rr=kCA1Akhdaw.com22R→Dr=kCD2RCA,CR为组分A及R的浓度。rA为组分A的转化速率,rD为D的生成速率。原料中3-13A的浓度等于0.1kmol/m,反应温度下,k1=0.1min,k2=1.25m/kmol.min,试计算反应器出口处A的转化率及R的收率。解:V2.2.02.00τ=r课后答案网==40min440min0minQ0.50.50CXXA0AAτ=www.hackshp.cn=kC(1−X)0.1(10.0.1(11(1−X)1A0AA所以:3X=0.800.80,,C=C(1−X)0.02=kmolm/AAA0ACτ=R=402kC−2kC1A2R即为:2100C+C−0.0800.0.080080=AR3C=0.023720.0230.0237272kmolm/RC0.023720.0230.0237272RY===0.23720.2370.23722RC0.10.1A03.16在全混流反应器中等温进行下列液相反应:22A⇔Br=kC−kCB1A2BAC+⎯⎯→k3Dr=kCCD3AC进料速率为360l/h,其中含25%A,5%C(均按质量百分率计算),料液密度等于30.69g/cm。若出料中A的转化率为92%,试计算:(1)(1)所需的反应体积;-5(2)(2)B及D的收率。已知操作温度下,k1=6.85×10khdaw.coml/mol.s;若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
-9-1;-5k2=1.296×10s;k3=1.173×10l/mol.s;B的分子量为140;D的分子量为140。解:因MB=MD=140,所以MA=MC=70QW0ρA3600.690.253600.3600.690.25×690.25×F===0.88710.8870.88711kmolh/A0M70AF0.8870.0.887110008871100011000×A0C===2.4622.46.46moll/A0Q3603600QWρ3600.690.053600.3600.690.05×690.05×0CF===0.17740.1770.17744kmolh/C0M70C0.1770.0.177410001774100041000×C==0.49290.4920.49299moll/A0360360CXτ=A0Akhdaw.com22(2(kC−kC)+kCC1A2B3AC(1)Cτ=B2kC−kC1A2B(2)C−Cτ=C0CkCC3课后答案网AC(3)由(2),(3)式分别得:2kCτ1AC=Bwww.hackshp.cn1+kτ2(4)CC=C0C1+kCτ3A(5)将(4),(5)式及上述数据代入(1)式,可整理为τ的代数方程式,解之得τ5=3.831×10s=106.4h3V=τQ=106.436038300106.4106.436038300×36038300=l=38.30m(1)(1)反应体积r0C=1.016moll/(2)(2)将τ代入(4)式得B,所以B的收率为:2C21.01621.021.016×16Y=B==82.60%82.6082.60%%BC2.462.2.4646A0C−C=2C+C对A作物料衡算:A0ABD所以有:C=C−C−2C=CX−2C=2.460.2.460.92×92−×21.016=0.2312moll/DA0ABA0ABC0.23120.2310.23122Y=D==9.40%99.40%.40%DC2.462.2.4646所以D的收率为:A03.17在CSTR中进行下述反应:khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
CH+Cl⎯⎯→k1CHCl+HCl66265()B()C(M)(H)(1)CHCl+Cl⎯⎯→k2CHCl+HCl652642(M)()C()(()D)(H)(2)CHCl+Cl⎯⎯→k3CHCl+HCl6422633()D()C()(()T)(H)(3)如果k1/k2=8,k2/k3=30,CB6=10.0mol/l,氯对苯的加料比=1.4,k1τ=1l/mol,(τ为空时),试计算反应器出口B,M,D,T,C的浓度。各个反应对各反应物均为一级。3C=10kmolmkk/,/=8,kk/=30,C/C=1.4,kτ=1解:B01223C0B01分别列出组分B,M,D,T,C的物料衡算式:khdaw.comC−CCB:τ=B0B,C=B0BkCC1+C1BCC(1)C8CCM:τ=M,C=BCMkCC−kCC8+C1BC2MCC(2)C30CCD:τ=课后答案网D,C=CMDkCC−kCC242402400+C2CM3DCC(3)C1T:τ=T,C=CCwww.hackshp.cnTCDkCC30303CD(4)CC:=C−C−2C−3CCC0MDT(5)由(5)式得:8CC60CC1BCCM1.41.4C=C+C+2C+3C=C+++CCB0CMDTCCD88+C240+C10CC(6)联立(1),(2),(3),(4),(6)式(五个方程,五个未知数):由(2)式得:8CC8CCBCB0CCM==8+C(1+C)(8+C)CCC(7)由(3)式得:230CC240CCC=CM=B0CD240+C(240+C)(1+C)(8+C)CCCC(8)将(1),(7),(8)式代入(6)得:238CC480CC24CC1.41.4C=C+B0C+B0C+B0CB0C(1+C)(8+C)(240((240240+C)(1+C)(8+C)(240+C)(1+C)(8+C)CCCCCCCC432C+475C+356235356262C−9232C−268800=整理得:CCCC3解得:CC=0.908kmol/mkhdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
103C==2.2.301301kmolm/B代入(1)式得:13.34513.3413.345+582.3013.34582.3082.3013.345×13.345×3C==5.427kmolm/M代入(7)式得:83.34583.3483.345+5303.3455.427303.3303.3455.427×455.427×3C==2.238kmolm/D代入(8)式得:2403.3452403.2403.345+34513C=×3.3452.2380.03123.3453.3452.2380.0312×2.2380.0312=kmolm/T代入(4)式得:301.41.4C=C+C+2C+3C验证:B0CMDT1.410×=3.345+5.427+×22.238+×30.031214.014.0=即:khdaw.com3.18根据例题3.12中规定的条件和给定数据,使用图解法分析此反应条件下是否存在多定态点?如果为了提高顺丁烯二酸酐的转化率,使原料以0.0013m/s的流速连续进入反应器,其它条件不变,试讨论定态问题,在什么情况下出现三个定态点?是否可能实现?解:由例3.11,3.12知:123−RA=课后答案网kCCAB=1.3711.371.3710exp(12628/)×10exp(12628/)0exp(12628/)−TCCkmolmsAB/.33T=326,KQ=0.0100.01.01m/,hV=2.65m00r33C=4.55kmolmC/,=5.34kmolm/A0www.hackshp.cnB03∆H=−33.5kJmol/=−33.510×kJkmol/r3ρCpt=1980kJmK/.移热速率方程:qr=Q0ρCpt(T−T0)19.8())19.8(=19.8(T−326)(1)放热速率方程:q=−∆((H)(−RV)grAr312=33.5133.510×0×1.3710exp(12628/)×−TC(1−X)(C−CX)2.65×A0AB0A0A(2)C(−∆H)A0rT−T=X0AρC绝热操作方程:pt(3)由(3)式得:ρCptX=(T−T)A0C(−∆H)A0r(4)(4)(4)代入(2)式得:⎡ρC⎤312ptq=33.533.5133.510×100×1.3710exp(12628/)×−TC⎢1−(T−T)⎥gA00⎢C(−∆H)⎥⎣A0r⎦⎡ρC⎤pt1515−42⎢C−(T−T)⎥×2.652.62.655=553.310exp(12628/)(128.80.6291×−T−T+7.677.6771071071710×0T)B00⎢(−∆H)⎥⎣r⎦khdaw.com(5)若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
由(1)式及(5)式作图得:T326330340350360362.5365367.5370qg44.163.87149.7314.8586.7670.8756.8851.8942.1由上图可知,此反应条件下存在着两个定态点。如果为了提高顺丁烯二酸酐的3’转化率,使Q0=0.001m/s,而保持其它条件不变,则这时的移热速率线如qr所示。’由图可知,qr与qg线无交点,即没有定态点。这说明采用上述条件是行不通的。从例3.11可知,该反应温度不得超过373K,因此从图上知,不可能出现三个定态点的情况。3.19根据习题3.3所规定的反应及给定数据,现拟把间歇操作改为连续操作。试问:(1)(1)在操作条件均不变时,丙酸的产量是增加还是减少?为什么?(2)若丙酸钠的转换率和丙酸产量不变,所需空时为多少?能否直接应用3.3中的动力学数据估算所需空时?khdaw.com(3)若把单釜操作改变三釜串联,每釜平均停留时间为(2)中单釜操作时平均停留时间的三分之一,试预测所能达到的转化率。解:(1)在操作条件均不变时,用习题3.3中已算出的Vr=4512l,Q0=57.84l/min,则可求出空时为τ=4512/57.84=78min。此即间歇操作时的(t+t0)。当改为连续操作时,转化率下降了,所以反应器出口丙酸的浓度也低于间歇反应器的结果。因Q课后答案网0维持不变,故最后必然导致丙酸的产量下降。这是由于在连续釜中反应速率变低的缘故。(2)若维持XA=0.72,则可由3.3题中的数据得出XA=0.72时所对应的反应速率,进而求出这时对应的空时τ=246.2min。因题意要求丙酸产量不变,故Qwww.hackshp.cn0不能变,必须将反应器体积增大至14240l才行。(3)这时τ1=τ/3=82.1min。利用3.3题中的数据,可求出RA~XA之关系,列表如下:XA00.10.20.40.60.8-RA0.17190.13910.10960.060160.023630故可用作图法求此串联三釜最终转化率。第三釜出口丙酸钠的转化率为:XA3=0.787。3.20根据习题3.8所规定的反应和数据,在单个全混流反应器中转化率为98.9%,如果再有一个相同大小的反应釜进行串联或并联,要求达到同样的转化率时,生产能力各增加多少?解:(1)二个300l全混串联釜,XA2=0.989,QXV=0A1=300300r1k(1(1−X)A1(1)QX(−X)V=0A2A1=300303003000r2k(1(1−X)A2(2)解得:XA1=0.8951代入(1)式求出此系统的体积流量:khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
3000.08(10.8951)3000.3000.08(10.8951)×08(10.8951)−Q==2.812/2.8122.812/ls/=168.7/minl0,20.89510.8950.89511Q=16.02/min16.0216.02/minl/min3.8题中已算出0,1。因为最终转化率相同,故生产能力增加168.7/16.02=10.53倍。(2)二个300l釜并联,在最终转化率相同时,Q0增加一倍,生产能力也增加一倍。33.21在反应体积为0.75m的全混流反应器中进行醋酐水解反应,进料体33积流量为0.05m/min,醋酐浓度为0.22kmol/m,温度为25℃,出料温度为36℃,该反应为一级不可逆放热反应,反应热效应等于-209kJ/mol,反应速率常数与温度的关系如下:-7-1k=1.8×10exp(-5526/T),min3反应物料的密度为常数,等于1050kg/mkhdaw.com,热容可按2.94kJ/kg.℃计算。该反应器没有安装换热器,仅通过反应器壁向大气散热。试计算:(1)(1)反应器出口物料中醋酐的浓度;(2)(2)单位时间内反应器向大气散出的热量。解:3T=298,KT=309,3309,09,KQ=0.05m/min00∆H=−课后答案网20910×3kJkmol/r7k=1.810exp(5526/309)0.3081.8101.810exp(5526/309)0.308×exp(5526/309)0.308−=(1)(1)求转化率:www.hackshp.cnQX0.050.0.0505X0AfAfV===0.750.0.7575rk(1−X)0.308(10.0.308(1308(1−X)AfAf解得:XAf=0.8221反应器出口物料中醋酐浓度:3C=C(1−X)0.22(10.8221)0.03914)0.)0.22(10.8221)0.03914=22(10.8221)0.03914−=kmolm/AA0A(2)单位时间内反应器向大气散出的热量:Q"=Q0ρCpt(T−T0)(+∆Hr)())((−RVA)r3=0.0510502.94(309298)(20910)0.3080.22(10.0.0510.0510502.94(309298)(20910)0.3080.22(10.0.0510502.94(309298)(20910)0.3080.22(10.8221)0.75×0502.94(309298)(20910)0.3080.22(10.×−+−××−8221)8221)0.750.75=−191kJ/min/m/minin33.22在反应体积为1m的釜式反应器中,环氧丙烷的甲醇溶液与水反应生产丙二醇-1,2:HCOCHCH+HO→HCOHCHOHCH23223-1该反应对环氧丙烷为一级,反应温度下反应速率常数等于0.98h,原料液中环3氧丙烷的浓度为2.1kmol/m,环氧丙烷的最终转化率为90%。(1)若采用间歇操作,辅助时间为0.65h,则丙二醇-1,2的日产量是多少?(2)有人建议改在定态下连续操作,其余条件不变,则丙二醇-1,2的日产量又是多少?(3)为什么这两种操作方式的产量会有不同?khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
3−1V=1mr,=kCk,=0.980.0.9898h,C=2.1kmollX/,=0.9解:rAAA0A(1)(1)一级不可逆反应:1111t=ln=ln=2.2.3535hk1−X0.9810.9−AfV=Qt(−t)=Q(2.350.65)1(2.(2.350.65)1350.65)1+=r0003所以Q0=0.109m/h3CX=2.10.91.892.10.2.10.91.89×91.89=kmolm/丙二醇的浓度=A0A丙二醇的产量=1.891/30.63×=kmolh/=15.15kmol/日(2)采用定态下连续操作QX0AfQ0×0.90.9V===1rk(1−X)0.98(10.9)0.0.98(10.9)98(10.9)−khdaw.comAf3所以Q0=0.109m/h丙二醇的产量=0.1091.890.2058×=kmolh/=4.939kmol/日(3)因连续釜在低的反应物浓度下操作,反映速率慢,故产量低。3.23根据习题3.11所规定的反应和数据,并假定反应过程中溶液密度恒课后答案网3定且等于1.02g/cm,平均热容为4.186kJ/kg.K,忽略反应热随温度的变化,且为-2231kJ/kg乌洛托品,反应物料入口温度为25℃。问:(1)(1)绝热温升是多少?若采用绝热操作能否使转化率达到80%?操作温度为多少?www.hackshp.cn(2)(2)在100℃下等温操作,换热速率为多少?C=2.03moll/,ρ=1.021.1.0202kgl/解:A0(−∆H)2231=kJkg/(乌洛托品)=312.3kJmol/(乌洛托品)r=78.09kJmol/(氨)Cpt=4.186kJkgK/.C(−∆H)2.2.0378.09037×8.09λ=A0r==37.13337.137.13K(1)绝热升温:ρCpt1.024.1861.0241.024.186×.186由物料衡算式(见3.11解答):Xτ=A32⎡1.4210exp(3090/)(11.4211.4210exp(3090/)(1×0exp(3090/)(1−T⎤−X)(3.163.045−X)⎣⎦AA由热量衡算式得:T=298+37.13XA。联立求解可得:XA=0.8578>0.8,T=329.9K可见,绝热操作时转化率可以达到80%。(2)T0=298K,在T=373K下等温操作,由物料衡算式可求出转化率:430430Xτ==A323⎡1.4210exp(3090/373)(11.4211.4210exp(3090/373)(1×0exp(3090/373)(1−⎤−X)(3.163.045−X)⎣⎦AA所以有:XA=0.9052khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
由(3.87)式可得物系与环境交换的热量:qUAT=h(0−T)=Q0ρCpt(T−T0)+QCX0A0Af(∆Hr)−3−3=×310×1.024.186(373298)3101.1.024.186(373298)310024.186(373298)310×−+××2.030.905278.09××=0.53020.5300.53022kJs/由上式知TC>T,说明应向反应器供热。3.24某车间采用连续釜式反应器进行已二酸和已二醇的缩聚反应,以生产醇酸树酯。在正常操作条件下(反应速度,进出口流量等),已二酸的转化率可达80%。某班从分析知,转化率下降到70%,检查发现釜底料液出口法兰处漏料,经抢修后,温度流量均保持正常操作条件。但转化率仍不能提高,试分析其原因。如何使转化率提高到80%?解:根据上述情况,可能是反应器的搅拌系统有些问题,导致反应器内部存在死区或部分物料走了短路,这些均可导致反应器的效率降低,从而使转化率下khdaw.com降。44管式反应器管式反应器管式反应器管式反应器4.1在常压及800℃等温下在活塞流反应器中进行下列气相均相反应:CHCH+H→CH+CH6532664在反应条件下该反应的速率方程为:课后答案网0.5r=1.5CC,molls/.//..TH式中CT及CH分别为甲苯及氢的浓度,mol/l,原料处理量为2kmol/h,其中甲苯与氢的摩尔比等于1。若反应器的直径为50mm,试计算甲苯最终转化率为95%www.hackshp.cn时的反应器长度。解:根据题意可知甲苯加氢反应为恒容过程,原料甲苯与氢的摩尔比等于1,即:C=CC=C=C(1(1−X)T0H0,则有:THT0T示中下标T和H分别代表甲苯与氢,其中:5pT00.51.013100.0.51.0131051.01310××−33CT0==3=5.68105.685.6810×10kmolm/RT8.314108.3148.31410×10×1073−3F=QC=2/21=kmolh/=0.27810×kmols/T00T0所以,所需反应器体积为:XTdXTXTdXTVr=QC0T0∫0.500.5.5=QC0T0∫1.501.51.5CCTH01.5CT−2.52.2.52.55−30.950.0.9595dXT(10.95)(1(10.95)−0.95)−13=0.278100.0.2781027810×∫0−31.511.1.5.55=0.4329×=3.006m1.5(5.6810)(11.5(5.6810)1.5(5.6810)(1×(1−XT)1.51−3.00633.006.006=1531.11531.1531.11m2所以,反应器的长度为:0.05×3.14/433.14/4.14/44.2根据习题3.2所规定的条件和给定数据,改用活塞流反应器生产乙二醇,试计算所需的反应体积,并与间歇釜式反应器进行比较。khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
解:题给条件说明该反应为液相反应,可视为恒容过程,在习题3.2中已算出:Q=275.8/275.8/275.8/lhC=1.2311.231moll/0A0所以,所需反应器体积:XAdXAV=QCr0A0∫0kC(1((11−X)(C−CX)A0AB0A0AQX275.80.950A===818.68818.618.6lkC1−X5.21.23110.955.215.21.23110.95×.23110.95−A0A由计算结果可知,活塞流反应器的反应体积小,间歇釜式反应器的反应体积大,这是由于间歇式反应器有辅助时间造成的。534.31.013×10Pa及20℃下在反应体积为0.5m的活塞流反应器进行一氧化氮氧化反应:khdaw.com2432NOO+→2NOr=1.411.41.410×100CC,kmolms/.22NONOO23式中的浓度单位为kmol/m。进气组成为10%NO,1%NO2,9%O2,80%N2,若进气流量为30.6m/h(标准状况下),试计算反应器出口的气体组成。解:由NO氧化反应计量方程式可知此过程为变容过程,其设计方程为:V课后答案网XAdXrA=CA0∫42Q01.41011.410.410×CC01.410AB(A)示中A,B分别代表NO和Owww.hackshp.cn2。由题意可知,若能求得出口转化率,由(2.54)式得:νy−iyXi0A0Aνy=Ai1+δyXAA0A便可求出反应器出口气体组成。已知:∑νi1δ==−,y=0.10.100.10,0,,y=0.09AA0B0ν2A3−43Q=0.6(27320)/2730.6440.6(20.6(27320)/2730.6447320)/2730.644+=m/h=1.788810×m/s00.60.6−2F==2.677102.6772.67710×10kmolh/i022.42222.4.4−22.677102.6772.67710×10×0.1−33C==4.159104.1594.15910×10kmolm/A00.64400.644.644−22.677102.6772.67710×10×0.09−33C==3.743103.7433.74310×10kmolm/B00.64400.644.644所以,反应速率为:khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
221C((11−X)(C−CX)A0AB0A0Ar=1.41011.410.410×42A2(10.05(10.0(10.05−5X)(10.05−X)AA2−34(1−XA)(3.7432.078)(3)(3.7432.078.7432.078−XA)10×=1.41011.410.410×3(10.05(10.0(10.05−5X)A再将有关数据代入(A)式:−330.5140.510.5144.15910×44.15910×4.15910×XA(10.05−XA)=dX−4∫2A1.789101.7891.78910×100(1−X)(3.7432.078−X)AA(B)X=99.7%用数值积分试差求得:A因此,yA0−yXA0A0.0.1(10.1(10.997)1(10.997)−0.997)y===0.032%0.0320.032%%khdaw.comA10.05−X10.050.997−×A10.09−×0.10.99700.10.997.10.997×2y==4.227%4.2274.227%%B10.050.99710.0510.050.997−×0.9972课后答案网0.1+×0.10.9970.0.10.99710.997×2y==11.546%11.5411.546%6%NO210.050.99710.0510.050.997−×0.9970.80.8y=www.hackshp.cn=84.197%84.1984.197%7%N210.050.99710.0510.050.997−×0.997另:本题由于惰性气体N2占80%,故此反应过程可近似按恒容过程处理,也不会有太大的误差。4.4在内径为76.2mm的活塞流反应器中将乙烷热裂解以生产乙烯:CH⇔CH+H262425反应压力及温度分别为2.026×10Pa及815℃。进料含50%(mol)C2H6,其余为水蒸汽。进料量等于0.178kg/s。反应速率方程如下:dp−A=kpAdt−1式中pA为乙烷分压。在815℃时,速率常数k=1.01.0s,平衡常数4K=7.49107.4917.4910×0Pa,假定其它副反应可忽略,试求:(1)(1)此条件下的平衡转化率;(2)(2)乙烷的转化率为平衡转化率的50%时,所需的反应管长。解:(1)设下标A—乙烷,B—乙烯,H—氢。此条件下的平衡转化率可按平衡式求取:ppK=BHppAkhdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
νipi0−pA0XAνApi=1+yA0δAXAνiyi0−yA0XAνAyi=,pi=yiP1+yA0δAXA1+1-1yA0=0.55,δA==11yA0−yA0XA0.5(1−XAe)yA==1+yA0δAXA1+0.5XAeνByB0−yA0XAνAkhdaw.comyB=yH=1+yA0δAXA10−*0.5*XAe0.5*X−1Ae==1+0.5X1+0.5XAeAe22pB课后答案网pHyByHP0.5XAe4K===P=7.49*14pAyAP(1+0.5XAe)(1−XAe)XAe=www.hackshp.cn0.61若以1摩尔C2H6为基准,反应前后各组分的含量如下:CH⇔CH+HHO,,∑262422反应前10012平衡时1-XeXeXe12+Xe因此,平衡时各组分分压为:PXPXP(1(1−X)p=1e,p=1e,p=1eBHA2+X2+X2+Xeee将其代入平衡式有:(Xe)2×2.02610/2.02.02610/2610/×51−Xe=7.4910×42+X2+XeeX=0.61解此一元二次方程得:e(2)(2)所需的反应管长:首先把反应速率方程变为dp(A/RT)kpA3−=,,kmolms/.dtRT以保证速率方程的单位与物料衡算式相一致。已知:0.1780.50.1780.1780.5×0.5F==0.00370.0030.00377kmols/A0300.5180.5300.5300.5180.5×+180.5×X=0.5X=0.3050.0.305305AfAekhdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
代入物料衡算式有XAfdXAV=FrA0∫01−XPk(A)12+XRTA30.0030.0.00378.3141000378.3141078.31410×××10880.30500.305.3052+XA3=dX=0.130.0.1313m12.0261012.0212.02610×610×5∫01−XAA其反应管长:0.130.0.1313L==28.82828.8.8m2(0.0762)(0.07(0.0762)62)×3.14/454.5于277℃,1.013×10Pa压力下在活塞流反应器进行气固相催化反应:CHOH+CHCOOH→CHCOOCH+HOkhdaw.com2533252()A()B()(()P)()Q3催化剂的堆密度为700kg/m,在277℃时,B的转化速率为:−7−64.09610(0.38.885104.0964.09610(0.38.88510×10(0.38.88510+×p)(p−pp/9.8p)QBPQAr=,kmolkgs/.B−43600(11.515103600(3600(11.5151011.51510+×p)P式中的分压以Pa表示,假定气固两相间的传质阻力可忽略不计。加料组成为课后答案网23%B,46%A,31%Q(均为重量%),加料中不含酯,当XB=35%时,所需的催化剂量是多少?反应体积时多少?乙酸乙酯的产量为2083kg/h。解:由反应计量方程式知反应过程为恒容过程,将速率方程变为B组分转www.hackshp.cn化率的函数,其中:p=p((11−X),p=p−pXBB0BAA0B0Bp=pX,p=p+pXPB0BQQ0B0B为求各组分初始分压,须将加料组成的质量百分比化为摩尔百分比,即12.34%B,32.1%A,55.45%Q。于是有:5p=0.12341.013100.1230.12341.0131041.01310××B05p=0.32201.013100.3220.32201.0131001.01310××A05p=0.55451.013100.5540.55451.0131051.01310××Q0208320208383F==0.01880.0180.01888kmols/B03600880.35360083600880.35×80.35×将上述有关数据代入设计方程:XBdXW=FBB0∫0rB4W=1.01101.0111.0110×0kg采用数值积分便可得到所需的催化剂量:其反应体积为:4W1.01101.0111.0110×03V===14.51414.5.5mrρ700700khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
4.6二氟一氯甲烷分解反应为一级反应:2CHClFg()→CFg()+2HClg()224流量为2kmol/h的纯CHClF2气体先在预热器预热至700℃,然后在一活塞流反应器中700℃等温下反应。在预热器中CHClF2已部分转化,转化率为20%。若反应器入口处反应气体的线速度为20m/s,当出口处CHClF2的转化率为40.8%时,出口的气体线速度时多少?反应器的长度是多少?整个系统的压力均为1.013×5-110Pa,700℃时的反应速率常数等于0.97s。若流量提高一倍,其余条件不变,则反应器长度是多少?解:反应历程如下图所示:反应器预热器温度khdaw.comT0TinTf=TiN线速度u0uinuf转化率XA0=0XA,inXA,fy=1,δ=1/211/2/2该反应为变容过程,其中A0A,2A→B+2C""FA0=FA0(课后答案网1−XA0))=2(1−0.20)=1.6"νB"1FB0=−νFA0XA0=2*2*0.2=0.2A"FC0=0.4www.hackshp.cn"∴Ft0=2.2FAf=FA0(1−XAf))=2(1−0.408)=1.184νB1FBf=−FA0XAf=*2*0.408=0.408νA2FCf=2*0.408=0.816∴Ft=2.408ufFt2.408===1.0945u"F"2.20t0uf=1.0945*20=21.89m/s由(2.50)式知:Q=Q(1+yδX)0A0AAu=u(1+yδX)0A0AA由已知条件,且考虑温度的影响可算出转化率为零时的线速度:u202200Tu=in=0=18.181818.18/.18/ms/0Tin10.50.2010.5010.50.20+×.20Tin(1+yδX)A0AAin,T0其出口处气体线速度为:khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
Tju=u(1+yδX)=18.18(10.50.408)21.89/1818.18(10.50.408)21.89/.18(10.50.408)21.89/+×=msj0A0AAT0由设计方程计算出反应器长度:LXAfdX=VQ/=CArinAin,∫u01−XinAkCAin,10.51010.5+.5XA那么需求出以反应器入口为基准的出口转化率XAf。据XA=FA0-FA/FA0,可求出FA,in=1.6kmol/h,FAf=1.184kmol/h,所以,XAf=(1.6-1.184)/1.6=0.26。因而有:u⎡1⎤0.2620inL=⎢(10.5((10.5)ln10.5)ln+)ln−0.5X⎥=(0.4520.13)6.63−=mAk⎣1−X⎦00.9700.97.97A这是由于反应器的截面积没有固定,固定的是反应气体的线速度等条件,因此,当流量提高一倍时,而其余条件不变,则反应器的长度并不变,只是其截面积khdaw.com相应增加。4.7拟设计一等温反应器进行下列液相反应:AB+→Rr,=kCCR1AB22A→Sr,=kC课后答案网S2A目的产物为R,且R与B极难分离。试问:(1)(1)在原料配比上有何要求?(2)(2)若采用活塞流反应器,应采用什么样的加料方式?www.hackshp.cn(3)(3)如用间歇反应器,又应采用什么样的加料方式?解:对于复合反应,选择的原则主要是使目的产物R的最终收率或选择性最大,根据动力学特征,其瞬时选择性为:1S=121+2kC/kC2A1B由此式可知要使S最大,CA越小越好,而CB越大越好,而题意又给出R与B极难分离,故又要求CB不能太大,兼顾二者要求:(1)原料配比,如果R与B极难分离为主要矛盾,则除去第二个反应所消耗的A量外,应按第一个反应的化学计量比配料,而且使B组分尽量转化。(2)若采用PFR,应采用如图所示的加料方式,即A组分沿轴向侧线分段进料,而B则在入口处进料。(3)如用半间歇反应器,应采取B一次全部加入,然后慢慢加入A组分,直到达到要求的转化率为止。4.8在管式反应器中400℃等温下进行气相均相不可逆吸热反应,该反应的活化能等于39.77kJ/mol。现拟在反应器大小,原料组成及出口转化率均保持不变的前提下(采用等温操作),增产35%,请你拟定一具体措施(定量说明)。设气体在反应器内呈活塞流。解:题意要求在反应器大小,原料组成和出口转化率均保持不变,由下式:khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
XAdXVQ/=CAr0A0∫0kfX()A可知,Q0与反应速率常数成正比,而改变反应温度又只与k有关,所以,提高反应温度可使其增产。具体值为:397703397709770−Ae8.31488.314.314T210.35110.35+0.35Q/Q=k/k===1.35020121397703397709770−1Ae8.3146738.3148.314673×673解此式可得:T2=702.7K。即把反应温度提高到702.7K下操作,可增产35%。4.9根据习题3.8所给定的条件和数据,改用活塞流反应器,试计算苯酚的产量,并比较不同类型反应器的计算结果。解:用活塞流反应器:khdaw.comXAdX11VQ/=CA=llnnr0A0∫0kC((11−X)k1−XA0AA将已知数据代入得:0.300.3.311=lnQ0.080.0.080810.989110.989−0.9890Q=课后答案网0.319m3/min解得:0,所以苯酚产量为:FX=QCX=0.3193.20.9891.010.3190.3193.20.9891.01×3.20.9891.01×=kmol/minA0A0A0A=1.09941.0991.0994www.hackshp.cn×4kg/min=94.99kg/min由计算可知改用PFR的苯酚产量远大于全混流反应器的苯酚产量,也大于间歇式反应器的产量。但间歇式反应器若不计辅助时间,其产量与PFR的产量相同(当然要在相同条件下比较)。4.10根据习题3.9所给定的条件和数据,改用活塞流反应器,反应温度和原料组成均保持不变,而空时与习题3.9(1)的反应时间相同,A的转化率是否可达到95%?R的收率是多少?解:对于恒容过程,活塞流反应器所需空时与间歇反应器的反应时间相同,所以A的转化率是可以达到95%的。R的收率与间歇反应器时的收率也相同,前已算出收率为11.52%。4.11根据习题3.14所给定的条件和数据,改用活塞流反应器,试计算:(1)所需的反应体积;(2)若用两个活塞流反应器串联,总反应体积是多少?解:(1)用PFR时所需的反应体积:−0.5XAdXAQC0A0−0.50.5V=QC=⎡(1−X)−1⎤r0A0∫0kC1.1.511.5.55(1−X)1.50.50.0.55k⎣A⎦A0A−0.50.51.520001.5201.52000×00−0.50.53=⎡(10.95)(10.9(10.95)−5)−1⎤=1.47m0.50.15810.50.0.50.1581×1581⎣⎦(2)若用两个PFR串联,其总反应体积与(1)相同。4.12在管式反应器中进行气相基元反应:AB+→khdaw.comC,加入物料A为气相,若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
B为液体,产物C为气体。B在管的下部,气相为B所饱和,反应在气相中进行。54已知操作压力为1.013×10Pa,B的饱和蒸汽压为2.532×10Pa,反应温度34023℃,反应速率常数为10m/mol.min,计算A的转化率达50%时,A的转化速率。3如A的流量为0.1m/min,反应体积是多少?解:此反应为气相反应,从化学计量方程式看,是变容反应过程,但气相中pB为常数,故可看成恒容过程。假定为理想气体,其中:4353p=2.532.5322.53210210×10Pak,=100m/mol.min10=m/kmol.minB5p=(1.0130.2532)10(1.01(1.0130.2532)1030.2532)10−×Pap,=p(1−X)A0AA0ApB43−33C==2.53210/(8.314102.5322.53210/(8.31410×10/(8.31410××613)4.9710=×kmolm/BRT5p0.759810(10.7590.759810(1810(1×−X)AAC===0.0149(10.0140.0149(19(1×−X)A3Akhdaw.comRT8.314108.3148.31410×10×613当XA=50%时,A的转化速率为:5−33R=kCC=10×4.97104.94.9710710××0.0149(10.5)3.7×−=kmolm/.minAAB33Q=0.1m/min/m/mininQ=0.10.2532/0.75980.0330.10.0.10.2532/0.75980.033×2532/0.75980.033=m/min当0A时,0B3Q=Q+Q=0.133m/min所以,00课后答案网0A0B此时所需反应体积为:XAdXAQ0XAdXAV=QC=r0A0∫0kCCkC∫01−Xwww.hackshp.cnABBA0.1330.0.1331331−43=ln=1.85101.81.8510510×m=0.185l−354.97104.9714.9710×0×1010.5−4.13在一活塞流反应器中进行下列反应:A⎯⎯→k1P⎯⎯→k2Q-1-13两反应均为一级,反应温度下,k1=0.30min,k2=0.10min。A的进料流量为3m/h,其中不含P和Q,试计算P的最高收率和总选择性及达到最大收率时所需的反应体积。解:对一级连串反应可得如下关系是:Y=1⎡(1−X)k2/k1−(1−X)⎤P1−k/k⎣AA⎦21(A)若求最高收率,即令:∂Y/∂X=0PA,可得到:11(X)=−1(k/k)1−k2/k1=−1(0.10.0.10.11)1010.1/10.1/0.3−.1/0.30.3=0.8076Am210.30.3将(XA)m代入(A)式得最高收率:10.1/0.3(Y)=⎡(10.8076)(10.(10.8076)−8076)−(10.8076)−⎤=57.84%Pmaxmax10.1/0.310.1/10.1/0.3−0.3⎣⎦P的总选择性:khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
(Y)0.0.57845784S=Pmax==71.62771.621.620(X)0.80760.80.8076076Am达到最大收率时的反应体积为:(XAm)dX313V=QCA=ln=0.2700.275.2755mr0A0∫0kC(1−X)600.3060600.30×0.3010.8076−1A0A4.14液相平行反应:0.33AB+→Pr,=CCkmolm/.min.mi.minnPAB0.51.31.1.333aAB(+)→Qr,=CCkmolm/.min.m.mininQAB式中a为化学计量系数。目的产物为P。(1)(1)写出瞬时选择性计算式。(2)(2)若khdaw.coma=1,试求下列情况下的总选择性。33(a)(a)活塞流反应器CA0=CB0=10kmol/m,CAf=CBf=1kmol/m;(b)(b)连续釜式反应器,浓度条件同(a);(c)(c)活塞流反应器,反应物A和B的加入方式如下图所示。反应物A从反应器的一端连续地加入,而B则从不同位置处分别连续加入,3使得器内处处B的浓度均等于1kmol/m,反应器进出口处A的浓度分3别为19和1kmol/m课后答案网。www.hackshp.cn解:(1)设A为关键组分,目的产物P的瞬时选择性为:νArP0.3νCC1S=P=AB=P0.30.50.0.551.3−0.5(−R)CC+αCC1+αCCAABABAB(2)若a=1,求下列情况下的总选择性。(a)活塞流反应器,因为CA0=CB0,其化学计量系数相同,所以CA=CB,则有1S=P0.51+CA,因此1CA0110dCAS=(−SdC)==0.320.30.326266OPC−C∫CAfPA10110101−1∫11+C0.0.50.55A0AfA(b)连续釜式反应器11S=S===0.0.50.55OPPj0.51+C2A1S=P−0.531+CA(c)PFR,且B侧线分段进料,器内B的浓度均等于1kmol/m,则khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
119dCS==A=0.73200.732.732OP∫−0.50.519119191−111+CA44.15在活塞流反应器中等温等压(5.065×10Pa)下进行气相反应:−63A→Pr,P=5.925.95.923102310310×pkmolmA/.min−53A→2,Qr=1.777101.71.777107710×pkmolm/.minQA−63A→3,Rr=2.961102.92.961106110×pkmolm/.minRA式中PA为A的分压Pa,原料气含量A10%(mol),其余为惰性气体。若原料气处3理量为1800标准m/h,要求A的转化率达到90%,计算所需的反应体积及反应产物Q的收率。解:此反应为复合反应系统,一般需要多个物料衡算式联立求解,方能解决问题。但这里三个平行反应均为一级,可简化处理。其组分A的总转化速率khdaw.com为:1111−5−5R=r+r+r=(0.5922(0.59(0.592222+×1.777+×0.2961)10×P=1.579410×PAPQRAA2323又为变容过程:0.59220.5920.592221.7770.2961δ=δ+δ+δA课后答案网1231.57941.5791.5794421.5794×31.5794×δ=0,0,δ=1,δ=2δ=0.6875其中123,所以有A43p(1(1−X)5.0655.06510×10×0.1(1−X)5.06510(1×−X)www.hackshp.cnA0AAAp===A1+δyX10.68750.110.610.68750.1+8750.1×X10.06875+XAA0AAA18000.11800018000.1×.1F==0.13390.1330.13399kmol/minA022.46022.4622.460×0所需反应体积为:XAdXXA10.0610.010.06875+6875875XdXAAAV=F=FrA0∫01.5794101.5791.579410410×−5pA0∫01.579410×−5×5.06510(1×3−X)AA0.13390.1330.13399⎧⎡1⎤⎫3=⎨(10.06875)ln(10.0(10.06875)ln+6875)ln−0.068750.9×−0⎬=4.22m−2⎢⎥810×⎩⎣10.910.10.9−9⎦⎭νAr1−5Q×1.777101.7771.77710×10pνAY=Q=2=0.56260.5620.56266Q−5R1.5794101.5791.579410410×p产物Q的收率:AA4.16在充填钒催化剂的活塞流反应器中进行苯(B)氧化反应以生产顺丁烯二酸酐(MA):B⎯⎯→k1MA⎯⎯→k2COCOHO,,22B⎯⎯→k3COCOHO,,22这三个反应均为一级反应,反应活化能(kJ/mol)如下:khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
E1=70800,E2=193000,E3=124800指前因子(kmol/kg.h.Pa)分别为8A1=0.2171,A2=1.372×10,A3=470.85反应系在1.013×10Pa和704K等温进行。原料气为苯蒸汽与空气的混合气,其中含苯1.8%(mol)。现拟生产顺丁烯二酸酐1000kg/h,要求其最终收率为42%。假设(1)可按恒容过程处理;(2)可采用拟均相模型。试计算(1)苯的最终转化率;(2)原料气需用量;(3)所需的催化剂量。解:(1)由题意知:−dC/dτ=(k+kC)B13B,解之得:C=Ce−(k1+k3)τBB0或:khdaw.com1−X=CB=e−(k1+k3)τBCB0且:dCMA=kC−kC=kCe−(k1+k3)τ−kC1B2MA1B02MAdτ课后答案网解此一阶线性微分方程有:⎡k2⎤Y=CMA=k1⎡e−k2−e−(k11+k3)τ⎤=k1⎢(1−X)k1+k3−(1−X)⎥MACk+k−k⎣⎦k+k−k⎢BB⎥M0www.hackshp.cn132132⎣⎦已知:70800−8.3147048.3148.314704×704−4k=0.21710.2170.21711e=1.21210×kmolkghPa/..1193000193001930000−88.3148.318.3147044704×704−7k=1.372101.3721.37210×10e=6.55910×kmolkghPa/..2124800124801248000−8.3147048.3148.314704×704−7k=470.8e=2.58710×kmolkghPa/..3Y=0.420.0.4242MA代入上式化简得到:0.44660.2823=⎡(1−X)−(1−X)⎤⎣BB⎦X=83.45%通过试差求出:BY=n/n=F/F(2)原料气需用量。由收率定义知:MAMAB0MAB0F100010100000F=MA==24.29524.2924.2955kmolh/B0Y0.42980.4290.4298×8MAF=24.295/0.0181349.75=kmolh/总原料气为:t(3)欲使XB达到83.45%,所需催化剂量由物料衡算式求得:khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
XBdXBFB0XBdXBW=F=B0∫∫0(k+kp)(k+kp)0(1((11−X)13B13B0B24.2924.29551=ln=16296.3162916296.36.3kg−65(1.2120.2587)10(1.21(1.2120.2587)1020.2587)10+××1.01310××0.01810.8345−4.17(1)写出绝热管式反应器反应物料温度与转化率关系的微分方程;(2)在什么情况下该方程可化为线性代数方程,并写出方程。回答问题(1),(2)时必须说明所使用的符号意义;CHCH+H→CH+CH(3)计算甲苯氢解反应6532664的绝热温升。原料气温度为873K,氢及甲苯的摩尔比为5。反应热△H298=-49974J/mol。热容(J/molK)数据如下:H2:CP=20.786CH4:CP=0.04414T+27.87khdaw.comC6H6:CP=0.1067T+103.18C6H5CH3:CP=0.03535T+124.85(4)在(3)的条件下,如甲苯最终转化率达到70%,试计算绝热反应器的出口温度。解:(1)绝热管式反应器反应物料温度T与转化率XA的微分方程:w(−∆H)dT=A0rTrdXAMC课后答案网Apt(A)(−∆H)式中rTr为基准温度下的热效应;C为反应物料在基准温度下与反应温度ptT之间的热容;wA0为组分A的初始质量分率;MA为组分A的分子量。(2)如果不考虑热容Cwww.hackshp.cnpt随物料组成及温度的变化,即用平均温度及平均组成下C的热容pt代替,则积分(A)式得:T−T=λ(X−X)0AA0(B)式中:T0为反应入口;XA0为初始转化率:w(−∆H)λ=A0rTrMCApt此时(A)式化为线性方程。当XA0=0时,又可写成:T=T+λX0A(3)求绝热温升。已知T0=873K,XA0=0,A表示关键组分甲苯,其初始摩尔分率yA0=1/6,为计算方便将(B)式改写成:y(−∆H)T−T=A0rT00C"pt(C)"此时Cpt是以摩尔数为基准的。选入口T为基准温度,需求出反应热0(−∆H)r873,以转化1mol甲苯为计算基准,则有:298(−∆H)=4997420.786(298873)4994997420.786(298873)7420.786(298873)+−+(0.03535T+124.85)dTr873873∫873873298229898298+∫(0.04414(0.04(0.04414414T+27.87)dT+∫(0.1067T+103.18)dT=80468.2/Jmol873887373873khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
从基准温度T0到出口温度反应物料的平均热容为:"4411Cpt=CpH+CpCH+CpCH24666666(D)式中各组分热容为各组分从基准温度至出口温度的平均热容。其绝热温升:y(−∆H)1/1/680468.2680×468.2A0r873λ==""CptCpt(E)因为反应出口未知,所以需将(C),(D)及(E)式联立试差求解得:λ=222K(4)在(3)的条件下,当XA=0.7时,绝热反应器的出口温度:T=8732220.701028.4+×=Kj4.18氨水(A)与环氧乙烷(B)反应以生产一乙醇胺(M),二乙醇胺(D)及三乙醇胺,反应如下:khdaw.comNH+CHO⎯⎯→k1HNCHCHOH324222HNCHCHOH+CHO⎯⎯k2→HNCHCHOH()22224222HNCHCHOH()+CHO⎯⎯→k3NCCHCHOH()22224223反应速率方程为:课后答案网r1=kCC1AB,r2=kCC2MB,r3=kCC3DB该反应系在等温下进行,目的产物为一乙醇胺。(1)(1)请你提出原料配比的原则,并说明理由。(2)(2)选定一种合适的反应器型式和操作方式。www.hackshp.cn(3)(3)根据(2)的结果,说明原料加入方式。(4)(4)反应时间是否有所限制?为什么?解:(1)若提出原料配比原则,应分析其动力学特征。这里以B为关键组分,目的产物M的瞬间选择性:kC2M1−kCS=1AkCkC2M3D1++kCkC1A1A由此看出CA增大时,则S也增大,无疑,相对来说CB减少。也就是说配比原则是:允许的条件下,尽量使A过量。(2)根据(1)的结果,可选活塞流反应器,并使B从侧线分段进料,而A从进口进料,采用连续操作,如图所示:(3)加料方式如(2)中的图示。(4)反应时间即停留时间有限制,因为目的产物M为中间产物,存在最佳收率,为达到最大收率,须控制最佳反应时间。34.19现有反应体积为1m的活塞流反应器两个,拟用来分解浓度为33.2kmol/m的过氧化氢异丙苯溶液以生产苯酚和丙酮。该反应为一级不可逆反-1应,并在86℃等温下进行,此时反应速率常数等于0.08skhdaw.com。过氧化氢异丙苯溶若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
3液处理量为2.4m/min。试计算下列各种情况下过氧化氢异丙苯溶液的转化率。(1)(1)两个反应器串联操作;(2)(2)两个反应器并联操作,且保持两个反应器的原料处理量相同,即3均等于1.2m/min;(3)(3)两个反应器并联操作,但两者原料处理量之比为1:2,即一个为330.8m/min,另一个则为1.6m/min;3(4)(4)用一个反应体积为2m的活塞流反应器替代;3(5)(5)若将过氧化氢异丙苯的浓度提高到4kmol/m,其余条件保持不变,那么,上列各种情况的计算结果是否改变?相应的苯酚产量是否改变?(6)(6)比较上列各项的计算结果并讨论之,从中你得到哪些结论?解:(1)两个反应器串联操作如图示:总反应体积为:khdaw.comXA1dXXAfdXXAfdXV=V+V=FA+FA=FArr11r2A0∫0A0∫XA0∫0(−RA)A1(−RA)(−RA)将有关数据代入即得:−0.082×X=−1e−kτ=−1e2.4/602.4/62.4/600=98.17%98.98.17%17%Af课后答案网(2)结果同(1)。3Q=0.0.88m/min/m/minin(3)第一个反应器,01,www.hackshp.cn−0.08X=−1e0.8/600.8/60.8/600=0.99750.990.997575A13Q=1.61.1.66m/min/m/minin而第二个反应器,02,−0.08X=−1e1.6/601.6/61.6/600=0.95020.950.950202A2两个反应器出口混合后:12X=×0.99750.9970.99755+×0.95020.9660=A333(4)用一个反应体积为2m代替,其结果同(1)。−kτ(5)当C提高到4kmool/m3时,由XAf=−1e可知,转化率与C无关,A0A0所以,上列各种情况计算结果不变,而对苯酚产量:(以摩尔流量表示)F=FX=QCXA0A0A0A说明苯酚产量与CA0成正比,即产量增加。(6)从上列各种计算结果比较看出:(a)几个PFR串联与用一个大的PFR,只要保持二者的总体积相同,其效果是一样的。(b)在并联时,只要保持V/Q=V/QV/Q≠V/Qr101r202,其结果也是相同的。但r101r202时,其总转化率是下降的。(c)对一级反应最终转化率与CA0无关,但目的产物的产量与CA0成正比关系。4.20在活塞流反应器中绝热进行丁二烯和乙烯合成环乙烯反应:khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
CH+CH→CH4624610该反应为气相反应,反应速率方程为:r=kCCAAB7k=3.1610exp(13840/),/3.1613.1610exp(13840/),/×0exp(13840/),/−Tlmols.5进料为丁二烯与乙烯的等摩尔混合物,温度为440℃。操作压力1.013×10Pa。5该反应的热效应等于-1.256×10kJ/mol。假定各气体的热容为常数,且CPA=154,CPB=85.6,CPR=249,单位为J/mol.K。要求丁二烯的转化率达12%,试计算(1)(1)空时,平均停留时间及出口温度;(2)(2)若改在440℃下等温进行,重复(1)的计算;(3)(3)440℃下等温反应时所需移走的热量。解:(1)此反应为绝热变温变容反应,空时:2VrXAdXA1XA(1(1+δAyXA0A)dXAτ==C=A0∫∫22khdaw.comQ0kCCC0k(1−X)(TT/)//))0ABA0A0平均停留时间:VV1XA((1(1+δyX)dXt=r=r=AA0AA∫2QQ(1+δyX)(/TT)C0k(1−X)(TT/)0AA0A0A0A0T=T+λXy=0.5,C=C,δ=−12/11212/1/1=−1出口温度:课后答案网0A,已知:A0A0B0A,∆H并假定选入口温度440℃为基准温度,题给r为440℃下的热效应。若以1molA为基准,则:Awww.hackshp.cn+B→R∑反应前1102XA1-XA1-XAXA2-XA所以,当XA=0.12时,y=y=(10.12)/(20.12)0.468(10.1(10.12)/(20.12)0.468−2)/(20.12)0.468−=ABy=0.12/(20.12)0.0640.12/0.12/(20.12)0.064(20.12)0.064−=RCpt=1540.46885.60.4682290.064128.1/1540.1540.46885.60.4682290.064128.1/×46885.60.4682290.064128.1/+×+×=JmolK.5−∆H=1.256101.2561.25610×10Jmol/r5y(−∆H)0.51.256100.0.51.2561051.×25610×A0rλ===521.55521.521.5Cpt(1(1+δAyXA0A)128.1(10.50.12)128.1128.1(10.50.12)(10.50.12)−×5pA01.013101.0131.01310×10×0.5−33C===8.544108.5448.54410×10kmolm/A03RT8.314108.3148.31410×10×713将数据代入并用数值积分得:khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
210.120.0.1212(10.(10.5−5X)dXAAτ=8.544108.5448.5441010−3∫013840713771313×7223.1613.1610exp(×0exp(−)(1−X)()A713521.571352713521.5+1.5X713521.5+XAA=68.16868.1.1s10.1200.0.12.1212(10.5(10.(10.5−5X)dXAAt=−3∫8.544108.5448.54410×1003.1613.1610exp(×0exp(7−13840)(1−X)(2713771313)A713521.571352713521.5+1.5X713521.5+XAA=67.56767.5.5sT=273440521.50.27344273440521.50.+0521.50.+×12775.6=K(2)在440℃下等温反应kkhdaw.com=3.3.1613.1610exp(13840/713)16×10exp(13840/713)0exp(13840/713)7−=0.117210.12(10.5(10.(10.5−5X)dXτ=AA8.544108.5448.54410×10−3×0.117∫0(1−X)2A1⎡(10.5)(10.(10.5)−5)2⎤0.120.0.1212=⎢2(0.5)(10.5)ln(12(0.52(0.5)(10.5)ln(1−)(10.5)ln(1−−X)(0.5)+−2X+XA⎥=128s−3AA8.544108.5448.54410×10×0.117⎣1−X⎦0课后答案网A10.120.0.120.12(10.5(10.(10.5−5XdX)t=AA−3∫28.544108.5448.54410×10×0.1170(1−X)A1⎡(10.5)(10.(10.5)−5)X1⎤0.120.0.1212=www.hackshp.cn⎢A+0.5ln⎥=113232s−38.544108.5448.54410×10×0.117⎣1−X1−X⎦0AAT=44027371344027440273713+3713=K(3)440℃等温反应,需移走热量,如果忽略由于反应造成的各组分的变化所引起的热容量变化,则若维持等温反应必须移走反应所放出的热量:q=−∆(H)(−RdV)=−∆(H)FdXr713Arr713A0A54=1.256101.2561.25610×10×0.12F=1.50710×FJh/A0A0其中FA6的单位为mol/h4.21环氧乙烷与水反应生成乙二醇,副产二甘醇:CHO+HO⎯⎯→k1CHOHCHOH224222CHOCHOHCHOH+⎯⎯→k2(CHCHOHO)2422222这两个反应对各自的反应物均为一级,速率常数比k2/k1为2,原料中水与环氧乙烷的摩尔比为20,且不含产物。(1)(1)选择何种型式的反应器好?(2)(2)欲使乙二醇的收率最大,转化率为多少?(3)有人认为采用活塞流反应器好,乙二醇收率高但环氧乙烷转化率低,故建议采用循环反应器以提高总转化率,你认为这种建议是否可行?如果循环比ψ=25,并使空时与第(2)问的空时相等,则此时总转化率及乙二醇的收率是提khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
高还是降低?解:(1)为解决问题方便,选H2O(B)为关键组分,环氧乙烷,乙二醇分别用A和Q表示。则乙二醇的瞬时选择性为:kC2QS=−1kC1B分析可知欲使S↑必须使CB↑,即使H2O过量,因而选PFR,且水从反应器入口进料,而环氧乙烷从侧线分段进料,相对来讲可使CB更大。(2)对水,乙二醇,二甘醇(E)为连串反应,存在最大收率(对乙二醇而言),此反应为液相反应可视为恒容过程,根据速率方程有:−dCkCQ2Q=−1dCkCB1B(A)C=C(1−X),C=CY因khdaw.comBB0BQQ0Q,故上式又可写成:dYkYQ2Q=−1dXk(1(1−X)B1B(B)初始条件XB=0,YQ=0,解此一阶线性常微分方程有:Y=1⎡(1−X)k2/k1−(1−X)⎤Q1课后答案网−k/k⎣BB⎦21(C)∂YQ=0∂X令Bwww.hackshp.cn有:X=−1(k2)1/(1−k2/k1)=−121/(121/(12)−)=0.5Bmk1本题给原料中水与环氧乙烷的摩尔比为20,其转化率是不可能达到这最佳转化率的。但它告诉我们,当CA=0时,为乙二醇的最大收率,即:C=C−⎡CX+C(X−Y)⎤=0AA0⎣B0BB0BQ⎦(D)CC121=2B0X−B0YBQCC解:A0A0CB0=20C又因为:nB:nA=20:1,所以有A0代入可得:Y=2X−0.05QB(E)将(E)式代入(C)式化简后可得:2X+X−0.05000.050.050=BB解此一元二次方程得:XB=0.048,也就是说欲使乙二醇收率最大,关键组分水的转化率为4.8%。(3)有人建议采用循环反应器,以提高转化率是不行的,因为增加了返混,降低了反应速率,反而使XB降低。如Ψ=25时,可视为CSTR,当空时与(2)中的khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
空时相等时,使总转化率下降,且使乙醇收率降低。4.22有一自催化液相反应A→P,其速率方程为R=kCCkmolm/3.min3AAP,反应温度下,k=1m/kmol.min,.mi.min,n,,3C=2kmolm/A0每小时处理1000mol原料,其中A占99%(mol),其余为P。要求最终转化率为90%。(1)(1)为使所需反应体积最小,采用何种型式反应器好?并算出你所选用的反应体积。(2)(2)如果采用循环反应器,请确定最佳循环比及反应体积。(3)(3)当循环比ψ=∞时,反应体积为多少?(4)(4)当循环比ψ=0时,反应体积为多少?解:(1)从自催化反应动力学特性可知,速率RA随CA的变化存在极大值,khdaw.com∂−(RA)=kC(+C))2))2−2kC=0A0P0A∂C令:ACA0+CP02020.02220.02+0.02.023(C)===1.01kmolm/A∞所以22(X)=−1C/C=−11.01/2111.01/21.01/2=0.495或:A∞课后答案网AA0其最大速率:3(−R)=kCC=kCC(+C−C))1.0)1.01(2.021.01)1.02=1.01(2.021.01)1.021(2.021.01)1.02−=kmolm/.minA∞APAA0P0A故采用两器串联可使反应体积最小,如图示,以极值处为两器的分界线。www.hackshp.cnF(X)1010.9910.990.495×.990.495×0.495V=A0A∞=r1kC(1−X)(C+CX)6602(10.495)(0.0220.495)02(10.495)(0.0220.495)×−+×A0A∞P0A0A∞3=0.00800.008.008mXAfdXF00.90.9dXV=FA=A0Ar2A0∫∫XA∞2(1−X)(0.022)()(0.0220.022+X)22×0.4950.00.495.495495(1−X)(0.01+X)AAAA0.9910.01+XA0.90.93=ln=0.0090.000.0099m6041.016041.6041.01×011−X0.49500.495.495A3V=V+V=0.0.0080.0080.0090.0170080.0090.017+0.0090.017=m所需最小总体积:rr1r2(2)如采用循环反应器,如图示:其基本设计式:XAfdXXAfdXV=F(1((11+ψ)A=F(1+ψ)ArA0∫A0∫2XA1(−R)XA1kC(1−X)(β+X)AA0AA(A)式中β=CP0/CA0=0.01,为求最佳循环比,令:∂VXAfdX1dXr=FA−F(1(1+ψ)A1=0A0∫A0∂ψXA1(−R)−R(X)dψAAA1khdaw.com(B)若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
ψXAfX=A11+ψ又因为,所以dX(1(1+ψ)X−ψXA1=AfAf2dψ(1(1+ψ)(C)将式(C)代入式(B)整理得:XAfdX1A=(X−X)∫AfA1XA1(−R)−R(X)AAA1XAfdXAXAf−XA1=∫22XA1kC(1−X)(β+X)kC(1−X)(β+X)即:A0AAA0A1A1积分化简后得:khdaw.com1β+XAXAfXAf−XA1lnln=1+β1−XX((11−X)(β+X)AA1A1A1(D)ψXAfX=A11+ψ将代入(D)式,整理得:(β+X)1⎡+ψ(1−X)⎤(1+ψ)(1+β)X课后答案网Af⎣Af⎦Afln=∂y(1−X)⎡βψβ+(+X)⎤⎡βψβ+(+X)1⎤⎡+ψ(1−X)⎤Af⎣Af⎦⎣Af⎦⎣Af⎦www.hackshp.cn(E)β=0.01,X=0.9将Af代入(E)式化简得:0.91(10.1)+ψ0.909(1+ψ)ln=0.0.0010.0010.0910010.091+0.091ψ(0.010.91)(10.1)+ψ+ψψ=0.41ψ=0.41试差求得:,即为最佳循环比。所以,对(A)式积分得时的反应体积:V=FA0(1(1+ψ)ln0.9090.909(1(1+ψ)=0.991.41××3.210.0183.213.210.018=0.018m3r22kC(1+β)(0.010.91)(10.1)(0(0.010.91)(10.1).010.91)(10.1)+ψ+ψ602××1.01A0ψ=∞(3)当循环比时,即为CSTR,反应体积为0.990.90.9900.990.9×.93V==0.040.0.0404mr2602(10.9)(0.010.9)602(1602(10.9)(0.010.9)×−0.9)(0.010.9)+ψ=0(4)当循环比时,即为PFR,反应体积为XAfdXA0.990.01+XA0.90.93Vr=FA0∫2=2lnln=0.028m02(1−X)(0.01)()(0.010.01+X)602(10.01)×+1−X0AAA4.23在常压和2300℃下在管式反应器中进行纯度为90%的甲烷高温热裂解反应:CH⎯⎯→k1CH⎯⎯→k2CH⎯⎯→k3C+H424222()A()B()(()D)其速率方程为:khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
−dCA=kC1AdτdC1B=kC−kC1A2Bdτ2dCD=kC−kC2B3Ddτ其速率常数为:13−1k=4.5104.514.510exp(45800/),×0exp(45800/),exp(45800/),−Ts18−1k=2.610exp(20100/),2.6102.610exp(20100/),×exp(20100/),−Ts26−1k=1.710exp(15100/),1.7101.710exp(15100/),×exp(15100/),−Ts3试问:khdaw.com(1)(1)若以C2H4为目的产物,忽略第三步反应,C2H4的最大收率为多少?(2)(2)若考虑第三步反应,C2H4的最大收率是否改变?(3)(3)用图表示各组分浓度随空时的变化关系。(4)(4)若改变甲烷的进料浓度,产物分布曲线是否改变?(5)(5)若改变反应温度,产物分布曲线是否改变?若提高反应温度,C2H4的收率是增加还是减少?乙炔的收率是增加还是减少?课后答案网解:由化学反应计量关系式可知,本反应是一个复杂的变容过程,计算是较复杂的。但是,实际上该高温裂解反应,为得到中间目的产物,通常XA只有百分之几,所以为便于计算和讨论,本题可近似看成恒容过程。(1)(1)若忽略第三步,Cwww.hackshp.cn2H4的最大收率:5k1k/(/(k−k)8.37108.3710×1.0510/(8.37101.0510)1.0511.0510/(8.37101.0510)×0/(8.37101.0510)5×5−×5−0.143Y=()221=()=7.97=0.743Bmaxmax5k1.05101.0510×2(2)(2)若考虑第三步反应,C2H4的最大收率不变,与(1)相同。(3)(3)用图表示各组分随空时的变化关系,由动力学数据可导出C=Ce−k1τAA01CkA−kτ−kτC=20101(e2−e1)Bk−k121CkkCkkee−k3τ−e−k2τe−k3τ−e−k1τ2A0101012C=(−)Dk−kk−kk−k122313因题中未给出原料的其他组分,现假定原料中不含产物,则最终产物C或H2的浓度据物料衡算导出,如C(这里假定碳的拟浓度,用CC表示)C=C−C−2C−2CCA0ABDCCCY=−11A−2B−2D=X−Y−YCABDCCC或:A0A0A0因此,各组分Ci/CA0~τ的关系可据上式做出,其示意图如下:(4)若改变甲烷的进料浓度,产物相对浓度分布曲线趋势不变,但产物浓度的绝对量是变的。khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
(5)若改变反应温度,产物分布曲线改变。若提高反应温度,有利于活化能大的反应,所以C2H4的收率增加,而乙炔收率减少。4.24温度为1000K的纯丙酮蒸汽以8kg/s的流量流入内径为26mm的活塞流反应器,在其中裂解为乙烯酮和甲烷:CHCOCH→CHCO+CH3324该反应为一级反应,反应速率常数与温度的关系为:lnk=34.3434222/−T-1k的单位为s。操作压力为162kPa。反应器用温度为1300K的恒温热源供热,2热源与反应气体间的传热系数等于110W/mK,要求丙酮的转化率达20%。各反应组分的热容(J/mol.K)与温度(K)的关系如下:−62CHCOH:C=226.626.630.1836.630.18330.183+T−45.8610×T33p−62CHCOC:=20.040.094520.020.040.094540.0945+T−30.9510×Tkhdaw.com2p−62CH:C=13.390.07713.313.390.07790.077+T−18.7110×T4p298K时的反应热等于80.77kJ/mol。(1)(1)计算所需的反应体积;(2)(2)绘制轴向温度分布及轴向丙酮浓度分布图。解:此反应为非绝热变容变温反应。其中课后答案网T=1000;KT=1300;Kd=0.026;0.0.026;026;mM=58;F=8000/58137.93=mols/;0CtAA02U=110WmKs/..;(...;(.;(∆H)=80.77kJmol/r298298。现选入口温度T0为基准温度,需求出:www.hackshp.cn1000101000001000(∆H)=∆(H)+CdT+CdTCdTrT0r298298∫298pA∫298pB∫229898pC(∆H)=81544.4/Jmol将有关数据代入得rT0然后将所需数据代入基本设计方程得微分方程组:dXA6(1(1))=3.84710(3.3.84710(84710(×−R)AdzdT43.27243.2743.27228.980(2)=−(−R)+(T−T)ACdzFCFCtpttpt161-X1-XA(3)(−R)=1.59710exp(-34222/T)11.59710exp(-34222/T).59710exp(-34222/T)×A1X+Az=0,00,,T=T,X=00Aδ=1,y=1,FC=FC式中AA0tpt∑ipi,此方程组用数值法求解。这里为方便学生用手算采用改良欧拉法,计算结果列入表中:丙酮裂解反应器的轴向XA和CA及T的分布T,KZ,mXACA,mol0.00.019.4851000.0100.00.009719.296khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
1003.5200.00.020419.0881006.3500.00.064318.2321018800.00.112217.29910271100.00.165816.2541032.21400.00.220515.189达到20%转化率所需反应体积,可按线性内插法确定反应器轴向长度:14001100−L=1400−(0.22050.1658)1400112.431287.57(0(0.22050.1658)1400112.431287.57.22050.1658)1400112.431287.57−=−=m0.22050.16580.2200.22050.165850.1658−再求反应体积:2π23.1403.143.140.026×0.026.0263V=dL=×1287.571287.1287.5757=0.6833mrt44显然,气体流过如此长的管道压力降是不可忽略的。而上述计算是基于等压情况处理的,较准确的计算应考虑压降的影响,应再加一个动量衡算式,然后再khdaw.com联立求解。不过,实际上如此长的管道是不可取的,应考虑很多根管子并联,即列管式反应器,这里不再多述。(2)可根据表中数据绘制轴向反应温度及丙酮浓度分布图。(这里从略)4.25在等温活塞流反应器中进行一级不可逆反应,正逆反应的反应速率常����数k和课后答案网k与温度的关系如下:��65000��9900090900000k=×210exp(210ex210exp(p(−),k=3.510exp(×−)TT要求最终转化率为90%,试问在什么温度下操作所需的反应体积最小?www.hackshp.cn解:由(2.31)式:����1E−E=∆Hrν��������可知,当E=5000,RE=9000R时,即E≻E,所以∆Hr≺0,故可判定此反应为可逆放热反应。据式(2.37)TT=e��OPRTE1+��e��ln��E−EE求最佳温度,其中Te为对应于转化率为90%时的平衡温度,对一级可逆反应有:����E−E900050009000590005000−000T=��==413.94413.913.9Ke9AX3.510×0.9Rln[lnln[[��A]ln[ln[]6A1−X210×10.91010.9−.9A413.9T==390.23390.290.2KOP413.990001+ln所以:900059900050000005000−0005000即,在390.2K下操作所需的反应体积最小。55停留时间分布与反应器停留时间分布与反应器停留停留时间分布与反应器时间分布与反应器khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
5.1设F(θ)及E(θ)分别为闭式流动反应器的停留时间分布函数及停留时间分布密度函数,θ为对比时间。(1)(1)若该反应器为活塞流反应器,试求(a)(a)F(1)(b)E(1)(c)F(0.8)(d)E(0.8)(e)E(1.2)(2)若该反应器为全混流反应器,试求(a)F(1)(b)E(1)(c)F(0.8)(d)E(0.8)(e)E(1.2)(3)若该反应器为一个非理想流动反应器,试求∞∞∫E()θdϑ∫θE()θdϑ(a)F(∞)(b)F(0)(c)E(∞)(d)E(0)(e)0(f)0解:(1)因是活塞流反应器,故符合理想活塞流模型的停留时间分布,由(5.33-5.36)式可得:(a)F(1)=1.0(b)E(1)=∝(c)F(0.8)=0(d)E(0.8)=0(e)E(1.2)=0(2)(2)因是全混流反应器,故符合理想全混流模型的停留时间分布,由khdaw.com(5.33-5.36)式可得:-1-1-0.8(a)F(1)=1-e=0.6321(b)E(1)=e=0.3679(c)F(0.8)=1-e=0.5507-0.8(d)E(0.8)=e=0.4493(e)=E(1.2)=0.3012(3)(3)因是一个非理想流动反应器,故可得:∞∫E()θdϑ(a)F(∞)=1(b)F(0)=0(c)E(∞)=0(d)E(0)>1(e)0=1∞课后答案网∫θE()θdϑ(f)0=15.2用阶跃法测定一闭式流动反应器的停留时间分布,得到离开反应器的示www.hackshp.cn踪剂与时间的关系如下:⎧0t≤2⎪ct()=⎨t−22≤≤t3⎪⎩1t≥3试求:(1)(1)该反应器的停留时间分布函数F(θ)及分布密度函数E(θ)。2σ(2)(2)数学期望θ及方差θ。(3)(3)若用多釜串联模型来模拟该反应器,则模型参数是多少?khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
(4)(4)若用轴相扩散模型来模拟该反应器,则模型参数是多少?-1(5)若在此反应器内进行一级不可逆反应,反应速率常数k=1min,且无副反应,试求反应器出口转化率。解:(1)由图可知C0=C(∝)=1.0,而F(θ)=F(t)=C(t)/C(∝),所以:⎧0t≤2,ϑ≤08.⎪F()θ=Ft()=⎨t−22≤≤t3,08.≤θ≤12.⎪⎩1t≥3,θ≥12.如下图所示:由(5.20)式可得平均停留时间:∞11t=∫0tEtdt()=∫0tdFt()=∫0[Ft()(())+2dFt()]=25.min即为上图中阴影面积。由(5.5)式得:khdaw.com⎧0t≺2dFt()⎪Et()(())==⎨12≤≤t3dt⎪⎩0t≻3所以:课后答案网⎧0ϑ≺08.⎪E()θ=tEt()=⎨25.08.≤θ≤12.⎪www.hackshp.cn⎩0θ≻12.如右图所示:t=VQ/=τ(2)由于是闭式系统,故r,所以θ=1由式(5.23)可得方差:∞212.222σ=θE()θdθ−θ=25.θdθ−=1001333.θ∫0∫08.2N=1/σ=100133375/.=(3)由(5.20)式可得模型参数N为:θ2σ≈2/Pe(4)(4)由于返混很小,故可用θ,所以:2Pe≈2/σ=2001333150/.=θ(5)用多釜串联模型来模拟,前已求得N=75,应用式(3.50)即可计算转化率:τN125×.75X=−111/(+k)=−111/(+)=09145.AN75同理,亦可用扩散模型即(5.69)式得XA=0.9146。两种方法计算结果相当吻合。5.3用阶跃法测定一闭式流动反应器的停留时间分布,得到离开反应器的示踪剂与时间的关系如下:t,s015253545556575901003C(t),g/cm00.51.02.04.05.56.57.07.77.7khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
(1)(1)试求该反应器的停留时间分布及平均停留时间。(2)若在该反应器内的物料为微观流体,且进行一级不可逆反应,反应速率常-1数k=0.05s,预计反应器出口处的转化率。(3)若反应器内的物料为宏观流体,其它条件均不变,试问反应器出口处的转化率又是多少?解:(1)由式(5.17)计算出反应器的停留时间分布,即:F(t)=C(t)/C(∝)=C(t)/7.7所得数据见下表所示:t,s01525354555657595100F(t00.0640.1290.2590.5190.7140.8440.9091.01.0)997532100将上述数据作图即为反应器停留时间分布。1t=∫tdFt()=×t1根据khdaw.com0由右图可知,可用试差法得到t,使两块阴影面积相等。由图试差得t=46s。(2)因进行的是一级反应,故可采用离析流模型预计反应器出口转化率。由式(3.12)可得间歇反应器中进行一级不可逆反应时转化率与反应时间的关X=−1exp(−kt)=−1exp(.005t)系:A代入离析流模型可得反应器出口处平均转化率:课后答案网∞11XA=∫0XEtdtA()=∫0XdFtA()=∫0[1−exexp(p(−005.tdFt)]()(A)采用图解积分法对(A)式进行积分,其中不同时间t下的F(t)如上表所示,www.hackshp.cn[1−exp(−005.t)]的值列于下表中:t01525354555657595100-0.1-e00.520.710.820.8940.9360.9610.9760.9910.99305t763562612533-0.05t以F(t)~(1-e)作图,计算积分面积得:X=845.%A(3)(3)由于是一级反应,所以混合态对反应速率无影响,故反应器出口"X=X=845.%转化率#与微观流体时相同,即AA。5.4为了测定一闭式流动反应器的停留时间分布,采用脉冲示踪法,测得反应器出口物料中示踪剂浓度如下:t,min012345678910C(t),g/l0035664.53210试计算:2(1)(1)反应物料在该反应器中的平均停留时间t和方差σθ。(2)(2)停留时间小于4.0min的物料所占的分率。解:(1)根据题给数据用(5.13)式即可求出E(t),其中m可由(5.14)式求得。本题可用差分法。khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
m=∑QCt()∆t=(356645321++++.+++)Qt∆=(356645321++++.+++)××1Q=305.QQCt()QCt()Ct()(())Et()===m305.Q305.然后按照(5.20)和(5.21)式算出平均停留时间和方差。此处用差分法,即:∑tEt()∆tt=∑Et()(())∆t(A)222σt=∑tEt()∆t−t(B)2σ为了计算khdaw.comt和θ,将不同时间下的几个函数值列与下表中:2tC(t)E(t)tE(t)△ttE(t)△t-1E(t)△t2ming/lminminmin000000100000230.098360.098360.19670.3934课后答案网350.16390.16390.49171.475460.19670.19670.79683.147560.19670.19670.98354.918www.hackshp.cn64.50.14750.14750.88505.310730.098360.098360.68854.8195820.065570.065570.52464.197910.032790.032790.29512.6561000000∑0.999884.85226.92将上述数据代入(A)和(B)式得平均停留时间和方差:t=48520999884853./.=.minmminin22σ=26924853.−.=3372.minmiminnt2222σ=σ/t=33724853./.=01432.θt(2)以E(t)~t作图(略),用图解积分法的:40.F(.)40=∫Etdt()=0362.0所以,停留时间小于4.0min的物料占的分率为36.2%。5.5已知一等温闭式液相反应器的停留时间分布密度函数-1E(t)=16texp(-4t),min,试求:(1)(1)平均停留时间;(2)(2)空时;(3)(3)空速;khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
(4)(4)停留时间小于1min的物料所占的分率;(5)(5)停留时间大于1min的物料所占的分率;(6)若用多釜串联模型拟合,该反应器相当于几个等体积的全混釜串联?(7)若用轴向扩散模型拟合,则模型参数Pe为多少?(8)若反应物料为微观流体,且进行一级不可逆反应,其反应速率常数为-16min,CA0=1mol/l,试分别采用轴向扩散模型和多釜串联模型计算反应器出口转化率,并加以比较;(9)若反应物料为宏观流体,其它条件与上述相同,试估计反应器出口转化率,并与微观流体的结果加以比较?解:(1)由(5.20)式得:∞∞−4tt=∫tEtdt()(())=∫t16tedt=05.min00(2)因是闭式系统,所以:khdaw.comτ==t05.min.min(3)(3)空速为空时的倒数,所以:11−1S===2minminντ05.1111−4t−4t−4tF()1=∫0Etdt()=∫016tedt=−4te+4∫0edt=09084.(4)课后答案网0所以,停留时间小于1min的物料所占的分率为90.84%。1−F()1=−10908400916.=.(5)www.hackshp.cn。停留时间大于1min的物料占9.16%。(6)先计算方差:∞∞2∞2222−2θσ=(ϑθ−)E()θdθ=ϑE()(()θ)dθ−θ=θ4θedθ−1θ∫0∫0∫03∞−2θ=−e−=105.20根据多釜串联模型参数与方差的关系得:11N===22σ05.θ2σ=05.(7)因θ,所以返混程度较大,故扩散模型参数Pe与方差关系应用:222−Peσ=−(1−e)θ2PePe采用试差法得:Pe=2.56。(8)因是一级不可逆反应,所以估计反应器出口转化率既可用扩散模型,也可用多釜串联模型或离析流模型,其结果应近似。采用多釜串联模型,由(3.50)式得:C111−X=A===016.AfN2C(1+kτ/N)(16052+×./)../)/)A0X=−1016084.=.所以有:Afkhdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
采用扩散模型,前已得到Pe=2.56,所以:05.05.α=(14+kτ/Pe)=(14605256+××./..././.)=2385.代入(5.69)式得:CA=4α/(⎨⎧1+α)ex)exp2p⎡−Pe((1−α)⎤−(1−α)exp2⎡−Pe(1+α)⎤⎬⎫⎢⎥⎢⎥CA0⎩⎣2⎦⎣2⎦⎭⎧2⎡25612385.(−.)⎤2⎡25612385.(+.)⎤⎫=×42385./(⎨12385+.)exp))expexp−−(12385−.)exp−⎬⎢⎥⎢⎥⎩⎣2⎦⎣2⎦⎭=01415.CX=−1A=−10141508585.=.AfC所以有:A0−6tCt()=Ce(9)用离析流模型,因一级不可逆反应,故间歇反应器的khdaw.comAA0,所以:CA∞CtA()∞−6t−4t∞−10t=∫Etdt()(())=∫e•16edt=∫16tedt=016.C0C00A0A0反应器出口转化率为XA=0.84,计算结果同前题用多釜串联模型与扩散模型结果相近。课后答案网5.6微观流体在全长为10m的等温管式非理想流动反应器中进行二级不可逆液相反应,其反应速率常数k为0.266l/mol.s,进料浓度Cwww.hackshp.cnA0为1.6mol/l,物料在反应器内的线速度为0.25m/s,实验测定反应器出口转化率为80%,为了减小返混的影响,现将反应器长度改为40m,其它条件不变,试估计延长后的反应器出口转化率将为多少?解:当反应器长度L=10m时,其空时为VL10rτ====40sQU025.kCτ=0266160401702..×.×=.A0已知有XA=0.80所以:1-XA=0.20。kCτ由上述A0与1-XA值,利用图5.23可查得:Da/UL=4。所以轴向有效扩散系数:2Da=4UL=×40251010.×=m/s当反应器长度改为40m,其空时应为L"40τ"===160sU025.kCτ""=0266160160.×.×=6810.所以,A0而反应器长度改变,轴向有效扩散系数Da值不变,所以:DaUL/"=10025401/./=kCτ"再利用图5.23,由A0与DaUL/"值查得:1-XA=0.060。所以反应器出口转化率应为:XA=1-0.060=0.94。khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
显然是由于反应器长度加大后,轴向返混减小,致使出口转化率提高。5.7在一个全混流釜式反应器中等温进行零级反应A→B,反应速率rA=9mol/min.l,进料浓度CA0为10mol/l,流体在反应器内的平均停留时间t为1min,请按下述情况分别计算反应器出口转化率:(1)(1)若反应物料为微观流体;(2)(2)若反应物料为宏观流体。并将上述计算结果加以比较,结合题5.5进行讨论。解:(1)因是微观流体,故可用全混流反应器的物料衡算式(5.24),且又是闭式系统,τ==t1min,所以:C−C10−Cτ=A0A=A=1R9A解得:khdaw.comCA=1moll/XA=−1CA//CA0=−1110/=090.(2)宏观流体且是零级反应,故只能用离析流模型(5.38)式,先确定式中CA(t)与t的关系。在间歇反应器中:−dCA=9mol/min.//min.min.ldt积分上式得:课后答案网⎧101009.−.tt≤CA⎪⎪9=⎨CA0www.hackshp.cn⎪0t≻10⎪⎩9上式中t=10/9min为完全反应时间。而全混流反应器的停留时间分布为:11Et()=exp(expexp((−t/)τ=exp(−tt/)τt代入(5.38)式中得:∞109/1"1−X=(C/C)()Etdt=(.1009−.)exp(t−ttdt/)=0396.A∫AA0∫00t"X=0604.所以出口转化率A由此可见,对于零级反应,其他条件相同,仅混合态不同,则出口转化率是不同的。且宏观流体的出口转化率为0.604,低于同情况下微观流体的出口转化率。但习题5.5是一级反应,所以混合态对出口转化率没有影响。5.8在具有如下停留时间分布的反应器中,等温进行一级不可逆反应A→P,-1其反应速率常数为2min。⎧0t≺1Et()=⎨⎩exexp(eexp(xp(p(1−t)t≥1试分别采用轴向扩散模型及离析流模型计算该反应器出口的转化率,并对计算结果进行比较。解:(1)用轴向扩散模型,故先确定模型参数Pe。为此需确定该反应器的khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
2σ停留时间分布特征--t与θ。∞∞t=∫tEtdt()=∫texp(expexp((1−tdt)=20.min00∞2∞2222σ=tEtdt()−t=texp(1−tdt)−tt∫∫00∞12222=∫texp(1−tdt)−∫texp(1−tdt)−t=−=541min002222σ=σ//t=12/=025.θt222−Peσ=−(1−e)=025.θ2而PePe迭代解得:Pe=6.8。代入(5.69)式中,得:12/12/khdaw.comα=(14+kτ/Pe)=(142268+××//.).)=1831.所以有:C41831×.A==00542.CA0(11831+.)ex)e)exp2xpp⎡−68.(11831−.)⎤−(11831−.)exp2⎡−68.(11831+.)⎤⎢⎥⎢⎥课后答案网⎣2⎦⎣2⎦反应器出口转化率为:XA=1-0.0542=0.9458(2)用离析流模型,对于一级反应:Ct()Awww.hackshp.cn=−(kt))exp)exp(=exp((−2t)CA0�歇所以:∞Ct()∞∞1−X=AEtdt()=exp(expexp((−2t)exp(1−tdt)=exp(13−tdt)=004511.A∫0∫1∫1CA0反应器出口转化率为:XA=1-0.04511=0.9549上述两种计算方法极为近似,这是由于在反应器中进行的是一级不可逆反应,混合态对其无影响。6.多相系统中的化学反应与传递现象6.1、在半径为R的球形催化剂上,等温进行气相反应A⇔B。试以产物B的浓度CB为纵座标,径向距离r为横座标,针对下列三种情况分别绘出产物B的浓度分布示意图。(1)(1)化学动力学控制(2)(2)外扩散控制(3)(3)内、外扩散的影响均不能忽略图中要示出CBG,CBSBBSS及CBeBBee的相对位置,它们分别为气相主体、催化剂外表面、催化剂颗粒中心处B的浓度,CBe是B的平衡浓度。如以产物A的浓度CA为纵座标,情况又是如何?解(1)以产物B的浓度为纵座标khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
(2)以产物A的浓度为纵座标khdaw.com课后答案网www.hackshp.cn6.2已知催化剂颗粒外表面的气膜传热系数为117w/m117w/m117w/m2K,气体的密度和热容分别为0.8k0.8kg/m0.8kg/m3和2.4J/kgK,2.4J/k2.4J/kgK,gK,试估算气膜的传质系数.解:2/32/31=J/J=(kρC/h)())((S/Pr),又S/Pr1=DHGpscc3−2k=h/ρC=117/0.82.410117/117/0.82.4100.82.410××=6.09410×ms/=219.4/mhGsp6.3某催化剂,其真密度为3.60g/cm3,3.60g/c3.60g/cm3,m3,颗粒密度为1.65g/cm3,1.65g/c1.65g/cm3,m3,比表面积为100m2/g.100m2/g.100m2/g.试求该催化剂的孔容,孔隙率和平均孔半径.解:由ρ=ρ(1−ε),得ε=0.54200.542.542ptpp由=2ε/Sρ,得=65.66565.6.6Ȧaprpa3由V=ε/ρ=0.542/1.650.3280.540.542/1.650.3282/1.650.328=cm/g催化剂gpp6.4已知铁催化剂的堆密度为2.7g/cm2.7g/cm2.7g/cm3,颗粒密度为3.83.8g/cmg/cmg/cm3,比表面积为16m2/g,试求每毫升颗粒和每毫升床层的催化剂表面积.解:2每毫升颗粒的表面积=ρS=60.8660.80.8m/mlpg2每毫升床层的表面积=ρS=43.2443.23.2m/mlbgkhdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
6.5试推导二级反应和半级反应的扩散有效因子表达式(6.23)(6.23)和((6.24).(6.24).解:(1):(1)二级反应,22α=2,22,,(−RA=)kCwAS,kaGm(CAG−CAS=kC)wAS22由上解得:CAS=−(kaGm±kaGm+4kkaCwGmAG)/2//22kw22按定义η=kC/kCxwASwAG2=−⎡kaGm(1±14+kCwAG/kaGm)/2//22kCwAG⎤⎣⎦2=(14+Da−1,)此即(6.23)式式中Da=kC/kakhdaw.comwAGGm(2)半级反应1/211/2/21/2α=1/2,1/2,((−RA)=kCwAS,kaCGm(AG−CAS=kCw)AS1/211/2/2222由上解得:C=−k±k+4kaC/2kaAS(wwGmAG)Gm1/21/21/1/22按定义:η课后答案网=kC/kCxwASwAG2221/2=−(k+k+4kaC)/2kaCwwGmAGGmAGwww.hackshp.cn1/21/22Da12⎛Da2⎞=−+Da+=4⎜1+−Da1+Da/4⎟22⎝2⎠1/21/2⎡22⎤⎢2+Da2(2+Da⎥)=−−1⎢24⎥⎢⎥⎣⎦1/21/2⎡⎛⎞⎤2⎢2+Da⎜4⎟⎥=1−1−⎢2⎜222⎟⎥⎣⎢⎜⎝(+Da⎟⎠⎥⎦)−1/21/2此即(6.24(6(6.24).24))式式中,:Da=kC/kawAGGm6.6在充填ZnO-Fe2O3ZnO-FZnO-Fe2O3e2O3催化剂的固床反应器中,进行乙炔水合反应:2CH+3HO→CHCOCH+CO+2H2223322已知床层某处的压力和温度分别为0.10Mpa,4000.10Mpa,4000.10Mpa,400℃,气相中C2H2含量为3%(mol),3%(3%(mol),mol),该反应速率方程为r=kCr=r=kCkCA,式中CA为C2H2的浓度,速率常数k=7.06k=7.06×107exp(-61570/RT),hexp(-61570/exp(-61570/RT),hRT),h-1,试求该处的外扩散有效因子.数据khdaw.com:催化剂颗粒直径0.5cm,0.5cm,0.5cm,若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
颗粒密度1.6g/cm1.6g/cm1.6g/cm3,C2H2扩散系数7.3×10-5m2/s/s,/s,,气体粘度2.35×10-5PaPPaa﹒ss,,床层中气体的质量流速0.2kg/0.2kg/m0.2kg/m0.2kg/mm2s.解:由已知条件可得2G=0.240.20.244kgms/⋅−5Re0.0050.24/2.3510Re0.00Re0.0050.24/2.3510=50.24/2.3510××=51.06(260.03180.97273260.03260.03180.97273×+180.97273×)3ρ=0.3303kgm/22.440027322.44022.4400273(0273+)0.359j=0.357/(Re0.357/0.357/(Re(Reε)0.2485=Db−5−5S=2.3510/(0.33037.310)0.97462.35102.3510/(0.33037.310)0.9746×/(0.33037.310)0.9746××=cGkkhdaw.comG=jD2/32/2/32/33=0.1837/0.18370.1837/ms/ρ(S)c7⎡−61615706615701570570⎤k=7.0610exp7.06107.0610exp×exp=1174.6111174.6/74.6/lh/=0.3263/ls⎢⎥⎣8.3146738.31468.314673×73⎦−33k=k/ρ=0.2039100.2030.203910910×mkgs/⋅wp课后答案网2⎡12⎤2am=π(0.005/)⎢π(0.005)×1600⎥=0.75mwww.hackshp.cn⎣6⎦Da=k/ka=0.001480.000.00148148wGm1η==0.99850.9980.99855π1+Da6.7实验室管式反应器的内径2.1cm,2.1cm,2.1cm,长80cm80cm,,内装直径6.35mm6.35m6.35mmm的银催化剂进行乙烯氧化反应,原料气为含乙烯2.25%(mol)2.25%(m2.25%(mol)ol)的空气,在反应器内某处测得P=1.06P=1.06×105Pa,TG=470K,==470K,=470K,乙烯转化率35.7%,环氧乙烷收率23.2%,已知14CH+O→CHO∆H=−9.61109.6119.6110×0JmolCH/2422412426CH+3O→CO+2HO∆H=−1.25101.251.2510×10JmolCH/24222224颗粒外表面对气相主体的传热系数为58.3w/m58.3w/m58.3w/m2K,颗粒密度为1.89g/1.89g/cm1.89g/cm1.89g/cm3.设乙烯氧化的反应速率为1.02×10-2kmkmol/kkmol/kgol/kgg﹒h,试求该处催化剂外表面与气流主体间的温度差.解:khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
(−R)10.2)10.)10.2=2molkgh/⋅A23.2⎛23.22323.2.2⎞5(−∆Hr)(=−∆H1)+−(∆H2⎜1)−⎟=55.00110.00110×Jmol/35.7⎝35.73535.7.7⎠252h=58.3wm/⋅=k2.09910×Jm/⋅Kh⋅s2Sπdpp2a===0.5000.50.50m/kgmVρ13ppπdρpp6T−T=−(R)(−∆H)/ha=48.59KgGArsm6.8一级连串反应:12khdaw.comA⎯⎯→B⎯⎯→C在0.1Mpa0.1Mpa0.1Mpa及360℃下进行,已知k-1--11-1--111=4.368=4.368ss,k2=0.417==0.417=0.417s0.417s,催化剂颗粒密度为1.3g/cm1.3g/cm1.3g/cm3,(k,(kGam)A和((kkGam)B均为20cm20c20cmm3/g//gg﹒ss..试求当CBGBBGG/C//CCAGAAGG=0.==0.=0.40.4时目的产物B的瞬时选择性和外扩散不发生影响时的瞬时选择性.解:外扩散无影响时,由(6.35)式得:0.41730.40.4170.41730.430.4×S′=−1=0.96180.960.961818课后答案网4.36844.368.368外扩散有影响时,由(6.34)式得:10.41730.4(1.168)0.410.41730.4(1.168)730.4(1.168)×S=−=0.94030.9400.9403310.0160510.0110.01605+www.hackshp.cn6054.368(1.01605)上式中所用的k/ρ1D==0.16800.168.168a1kaGmk/ρ2D==0.016050.0160.0160505a2kaGm6.9在Pt/AlPPt/Alt/Al2O3催化剂上于200℃用空气进行微量一氧化碳反应,已知催化剂的孔容为0.3cm3/g,//g,g,比表面积为200m2/g,//g,g,颗粒密度为1.2g/1.2g/cm1.2g/cm1.2g/cm3,曲节因子为3.7.CO-3.7.CO-3.7.CO-空气二元系统中CO的正常扩散系数为0.192c0.192cmm2/s.//s./s.试求CO在该催化剂颗粒中的有效扩散系数.解:r=2V/S=30,Ȧλ=10−5cm,ε=Vρ=0.36aggpgpλ/2r=16.610,16.16.610,610,≥为努森扩散故有,:a3−8−22Dk=9.71030109.7109.7103010×(3010×473/28)=1.19610×cm/s−32De=Dε/τ=1.164101.161.16410410×cm/skp6.10试推导球形催化剂的内扩散有效因子表达式(6.60).(6.60).khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
解:令C=u,可得:AdC1duuA=−2drrdrr22dC11du2du2uA=−+2223drrdrrdrr用以上各式对教材中(6.55)式进行变量置换得:2du2=bu2dr2式中b=kDep(A)(A)(A)(A)(A)(A)式为二阶常系数齐次微分方程,边界条件:khdaw.comr=0r=0du/dr=0;du/drdu/dr=0;=0;r=Rr=Rpu=Cu=CASRp(B(B)(B))结合边界条件(B)(B)式解(A)(A)得:Rsinh(br)pC=C()CAASrsinh(sisinh(nh(bR)p有内扩散影响时的反应速率为:(−R=)课后答案网De4πR2dCA()DApdrrR=pdC由()(()C)式求出A,代入()D式得:www.hackshp.cndrrR=p⎛11⎞(−RA=)4πRpφsCDeAS⎜−⎟tanhtatanhnhφφ⎝ss⎠按内扩散有效因子的定义:4πRpφsCDeAS⎛11⎞3⎛11⎞η=⎜−⎟=⎜−⎟()E3πRkC3⎝tanhtatanhnhφsφs⎠φs⎝tanhtatanhnhφsφs⎠ppAS4式中φ=bR=Rk/DespppRp若令=φφ/3=k/De,则()(()E)式可改写为:sp311⎛11⎞η=⎜−⎟()Fφ⎝tanh(tatanh(3)nh(3)3)φ3φ⎠(F)即为教材(6.60)(6.60)式,(,(E),(E)式是(6.60)的又一形式.6.11在球形催化剂上进行气体A的分解反应,该反应为一级不可逆放热反应.已知颗粒直径为0.3cm,0.3cm,0.3cm,气体在颗粒中有效扩散系数为4.5×10-5m2/h/h,/h,,颗粒外表面气膜传热系数为44.72w/m44.72w/m44.72w/m2﹒K,气膜传质系数为310m310m/h,310m/310m/h,/h,h,反应热效应为-162kJ/mol,-162kJ/mo-162kJ/mol,l,气相主体A的浓度为0.20mol/l,0.20m0.20mol/l,ol/l,实验测得A的表观反应速率为khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
1.67mol/minl,试估算:(1)(1)外扩散阻力对反应速率的影响;(2)(2)内扩散阻力对反应速率的影响;(3)(3)外表面与气相主体间的温度差.解:*53R=1.67mol/min//minmin⋅=l1.00210×molm/⋅hA(1)(1)判别外扩散阻力的影响用(6.79)式:3−3*1.67××101100RAL6−3==0.8081100.8080.808110110×<0.15Ck0.2(310/60)0.2(30.2(310/60)10/60)AGG故仅从传质考虑外扩散影响可不计,.(2)(2(2))判别内扩散阻力的影响用(6.82)(6.(6.82)82)式先求出,φ:khdaw.coms*2RLAφ==2.78322.783.783sDeCAG2因有φη=φ,从φ=2.783,2.782.783,3,可借助(6.60)式估算出φ=3.1,η=0.288,由此ss可知内扩散阻力影响严重.课后答案网(3)计算外表面与气相主体间温度差⊿Tm:*∆T=T−T=R⋅颗粒体积(H⋅∆)/h⋅颗粒外表面积=50.4KmGwww.hackshp.cnsArs6.12在固体催化剂上进行一级不可逆反应A→B()A已知反应速率常数k,催化剂外表面积对气相的传质系数为kGam,内扩散有效因子η.CAG为气相主体中组分A的浓度.((1)1)试推导:C(R)AG()B−A=11+kηkaGm(2)若反应式(A)(A)改为一级可逆反应则相应的(B)(B)式如何?解:(1):(1)一级不可逆反应AB:(−RA=)kGCAG(−CASam=ηk)CAS由上可解得:CAS=kaCGmAG/(ηk+kaGm)C解得:-R(=)ηkC=AGAAS1+kaGmηk(2)一级可逆反应A⇌B:khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
��由(−RA=)kaGmCAG(−CAS=η(k+)kC(AS)−CAe)kaC+ηkCGmAGAe解得:C=ASka+ηkGm��C−C则有:(−R=)η(k+kC()−C=)AGASAASAe11��+η(k+kka)Gm6.13在150℃,用半径100μm的镍催化剂进行气相苯加氢反应,由于原料中氢大量过剩,可将该反应按一级(对苯)反应处理,在内,外扩散影响已消除的情况下,测得反应速率常数kp=5min=5m=5minin-1,苯在催化剂颗粒中有效扩散系数为0.2c0.2cmm2/s,//s,/s,试问:(1)(1)(1)(1)((1)1)在0.1Mp0.1Mpa0.1Mpa0.1Mpaa下,要使η=0.8,催化剂颗粒的最大直径是多少?khdaw.com(2)((2)(2)(2)2)(2)((2)2)改在2.02Mp2.02Mpa2.02Mpa2.02Mpaa下操作,并假定苯的有效扩散系数与压力成反比,重复上问的计算.(3)(3)(3)(3)((3)3)改为液相苯加氢反应,液态苯在催化剂颗粒中的有效扩散系数10-5cmccmm2/s.//s./s.而反应速率常数保持不变,要使η=0.8,求催化剂颗粒的最大直径.解:dkpp(1)φ=课后答案网=0.10.1076076dp6De1⎛11⎞由(6.60)式η=⎜−⎟www.hackshp.cnφ⎜⎝tanh3tatanh3nh3(φ)3φ⎟⎠用试差法从上二式可解得当η=0.8时,需dp<<6.36cm<6.36cm(2)2.02Mpa((2)2.02Mpa2)2.02Mpa时,De≈0.2×0.101/2.02=0.010.101/2.02=0.010.101/2.02=0.01cmcm2/s,//s,/s,与此相对应:dkppφ==0.41800.418.418dp6De同上法可求得当η=0.8时,需dp<<1.42cm<1.42cm(3)液相反应时,D,De=1,De,De=1e=1=1×10-6cmccmm2/s,//s,/s,与此相应的φ为21.51dp,21.51dp,21.51dp,同上法可求得当η=0.8时,需dp<<0.0142cm.<0.0142c<0.0142cm.0.0142cm.m.6.14一级不可逆反应AB,在装有球形催化剂的微分固定床反应器中进行温度为400℃等温,测得反应物浓度为0.05kmol/m0.05k0.05kmol/mmol/m3时的反应速率为2.53床层﹒minmin,,该温度下以单位体积床层计的本征速率常数为k-1--11kmol/mkkmol/mmol/mv=50s,床层孔隙率为0.3,A的有效扩散系数为0.03cm0.03c0.03cmm2/s,//s,/s,假定外扩散阻力可不计,试求:(1)(1)(1)(1)((1)1)反应条件下催化剂的内扩散有效因子(2)((2)(2)(2)2)(2)((2)2)反应器中所装催化剂颗粒的半径khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
解:kV=kVppvBVV1BBk=k=k=k=71.43ls/pvvvVV−V1−εpBεdkppφ==8.138.8.1313dp6De3(−RA=)ηkCvAS=×η500.05500.0500.05×5kmolsm/⋅床层实验测得(-R(-RA)=0.0417)=0.0417kmol/skmol/skkmol/smol/s﹒m3床层,解上二式得η=0.0167,可见内扩散影响严重.由η==1/1/φ=1/8.13dp=0.0167,==1/8.13dp=0.0167,1/8.13dp=0.0167,可解出dp=7.38cm,dp=7.38cmdp=7.38cm,,即反应器所装催化剂的颗粒半径为3.69cm.3.69cm.3.69cm.khdaw.com6.15在0.10Mpa,5300.10Mpa,5300.10Mpa,530℃进行丁烷脱氢反应,采用直径5mm的球形铬铝催化剂,此催化剂的物理性质为:比表面积120m2/g,孔容0.35cm0.35c0.35cmm3/g,//g,g,颗粒密度1.2g/1.2g/cm1.2g/cm1.2g/cm3,曲节因子3.4.在上述反应条件下该反应可按一级不可逆反应处理,本征反应速率常数为0.94cm3/gs,//gs,/gs,外扩散阻力可忽略,试求内扩散有效因子.解:丁烷分子量为58,λ=10-5--55cmcm,cm,=2Vg/Sg=58.3,=2Vg/Sg=58.3=2Vg/Sg=58.3×10-8cmcm,cm,,λ/2=8.576,此值与10接近,故可近似扩散是以奴森扩散为主:课后答案网−8−22Dk=970059970058.31070058.310×8.310×(530273/58+)=2.10410×cm/s−32De=Dε/τ=22.612.610.610×0cm/swww.hackshp.cnkpm0.50.941.20.50.0.50.941.2941.2×φ==1.73611.736.736−362.6102.612.610×0由(6.60)式算得η=0.465.6.16在固定床反应器中等温进行一级不可逆反应,床内填充直径为6mm的2球形催化剂,反应组分在其中的扩散系数为0.02cm/s,在操作温度下,反应式速-1率常数等于0.01min,有人建议改有3mm的球形催化剂以提高产量,你认为采用此建议能否增产?增产幅度有多大?假定催化剂的物理性质及化学性质均不随颗粒大小而改变,并且改换粒度后仍保持同一温度操作.解:dkppd=0.6cm,φ==0.02887,0.0.02887,02887,η=0.9995,p6Ded=0.3cm,φ=0.01444,00.01444,.01444,η=0.9998p故采用此建议产量的增加是很有限的.6.17在V2O5/SiO2催化剂上进行萘氧化制苯酐的反应,反应在1.013×510Pa和350℃下进行,萘-空气混合气体中萘的含量为0.10%(mol),反应速率式为:khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
50.38⎛135360135361353600⎞r=3.821103.8213.82110×10pexp⎜−⎟,kmolkgh/⋅AA⎝RT⎠3式中PA为萘的分压,Pa.已知催化剂颗粒密度为1.3g/cm,颗粒直径为0.5cm,试计算萘氧化率为80%时萘的转化速率(假定外扩散阻力可忽略),有效扩散系数等-32于3×10cm/s.解:因外扩散阻力可不计,故CASAS≈CAAGG,0.380.0.3838(−RA=)ηkCpAGkmolkgh/⋅式中η由教材(6.66)式计算,为此先计算以下数据:−1−32aV/=12cm,De=×310331010cm/,sk的值由:ppp50.38⎛135360135361353600⎞r=3.82103.8213.8210×0pexp⎜−⎟kmolkgh/⋅AAG⎝RT⎠khdaw.com50.38⎛135360135361353600⎞3=3.82103.8213.8210×0ρpexp⎜−⎟kmolm/颗粒⋅hpAG⎝RT⎠将此P3颗粒,T=(350+273)K,T=(350+273),T=(350+273)KK代入上式,并将小时换为秒AG=C==CCAGAAGGRTRT,RT,,ρp=1300kg/m=1300k=1300kg/mg/m计则得:−40.383r=2.196102.1962.19610×10Ckmolm/颗粒⋅sAAG由上式得k课后答案网p=2.196×10--44又::CCASAASS=C==CCAGAAGG=P==PPAGAAGG/R/RT=10//RT=10RT=10T=105×0.1%(1-0.8)/(83140.1%(1-0.8)/(0.1%(1-0.8)/(83148314×623)=3.861623)=3.861623)=3.861×10-6kmkmol/mkkmol/mmol/mol/m3将有关数值代入(6.66)式得:apwww.hackshp.cn2DeCAS1/21/2η=[∫fC(AdC)A]=1.121.11.122VkfC()CACppAS0.38−3CAS1/1/22式中:fC(AS=)fCAG(=C)AG=8.76910[8.768.76910[910[×∫fC(AdC)dCA]CACCAS0.381/211/2/2−4=[CdC]=1.5663101.51.56631066310×∫0AA最后得萘氧化率为80%时的萘的转化速率为:0.380.0.3838−63(−RA=)ηkCpAG=2.157102.1572.15710×10kmolm/颗粒⋅s6.18乙苯脱氢反应在直径为0.4cm的球形催化剂上进行,反应条件是0.10Mpa,6000.10Mpa,6000.10Mpa,600℃,原料气为乙苯和水蒸汽的混合物,二者摩尔比为1:9,假定该反应可按拟一级反应处理.⎛913009913001300⎞r=kp′k′=0.1244exp0.1240.1244exp4exp⎜−⎟,kmol苯乙烯/kghPa⋅⋅wEBw⎝RT⎠(1)当催化剂的孔径足够大,孔内扩散属于正常扩散,扩散系数D’=1.5×10-5--55m2/s/s,/s,,试计算内扩散有效因子.(2)当催化剂的平均孔径为100Å时,重新计算内扩散有效因子.已知:催化剂颗粒密度为1.45g/cm1.45g/cm1.45g/cm3,孔率为0.35,曲节因子为3.0.解:为计算内扩散有效因子,先求取Kp:khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
3由r=kpkmolkghk/⋅=ρpkmolm/颗粒⋅hEBpEB将PEB=R=RTC=R=RTCTCEB,T=,T=(600+273),T,T=(600+273)=(600+273)(600+273)代入上式得:333r=4.504.5084.50810810×10Ckmolm/颗粒⋅=h1.252Ckmolm/颗粒⋅sBEEB由此得K-1--11p=1.252=1.252ss(1)((1)(1)(1)1)(1)((1)1)孔径足够大,属正常扩散时,−62De=Dε/τ=11.751.7510.7510×10m/spm由此求得Φ=0.564,=0.564,由((6.60)6.60)式算得η==0.850.85(2)(2)孔半径为100Å时:-5-8λ/2=10/200×10=5,属于过渡区扩散,由教材(6.36)式可算得乙苯-22的Dk=2.784×10cm/s.1−22D==2.2.3482.3481034810×10cm/s11khdaw.com−2+2.2.7842.7841078410×100.15Dεp−332−72De==2.739102.7392.73910×10cm/s=2.73910×m/sτm由上数据可算得φ=1.425,由教材(6.60)式算得η=0.528课后答案网6.19苯(B)在钒催化剂上部分氧化成顺酐(MA),反应为:www.hackshp.cn这三个反应均为一级反应.实验测得反应器内某处气相中苯和顺酐的浓度分别为1.27%和0.55%(均为mol%),molmol%),%),催化剂外表面温度为623K,此温度下,k,k1=0.0196,k1=0.01961=0.0196ss-1,k2=,k2=0.0158,k2=0.0158s0.0158s-1,k3=,k3=1.98,k3=1.981.98×10-3s-1--11,苯与顺酐的kGam均为1.0×10-4m3/s/skg./s/skg.kg.催化剂的颗粒密度为1500kg/m1500kg/m1500kg/m3,试计算反应的瞬间选择性并与外扩散无影响时的瞬时选择性相比较.解:−5−5−5由k=k/ρ可算出k=1.1.301.30710,30710,710,×k=1.05310,×k=0.13210×wpw1w2w33单位均为m/kgs⋅.由Da=k/ka可算得D=0.0.130.1307,1307,07,D=0.1053,D=0.0132wGma1a2a3为简化起见以表示顺酐,C表示(CO+COA),由教材(6.18)式可写出:2kGam(CBG−CBS=)kw1+(kw2CBS)(Α)kGam(CAS−CAG=kC)w1BS−kCw2AS(Β)ka(C−C=kC)−kC(C)GmCSCGw2ASw3BSkhdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
由(1)得C=C/(1+D+D)BSBGa1a2由(2)得C=C/(1+D)+DC/[(1+D+D)(1+D)]AsAGa22a1BGa1a2a1有外扩散影响时的瞬时选择性:S=(kCw1BS−kCw2AS)(/kw1+kw3CBS)kk⎡CDC⎤⎡1+D+D⎤w1w2AGa1BGa1a3=−⎢+⎥⎢⎥kw1+kw3kw1+kw3⎣1+Da2(1+Da1+Da3)(1+Da2⎦⎣)CBG⎦kk⎡(1(1+D+D)CD⎤w1w2a1a3AGa1=−⎢+⎥kw1+kw3kw1+kw3⎣(1+Da2C)BG1+Da2⎦=0.4740.470.4742422无外扩散影响时的瞬时选择性khdaw.com:kkCS′=w1−w2CG=0.57260.5720.57266k+kk+kCw1w3w1w3BG6.20原题见教材,今补充如下:实验测得A的气相浓度为1.68×10-5molmol/cmmmol/cmol/cm/cm3时的反应速率为1.04×10-5(m(mol/c(mol/cmol/cmm3床层﹒s).s).解:已知课后答案网*−5−53(−RA=1.0411.041.0410/(10.4)1.73310)×10/(10.4)1.733100/(10.4)1.73310−=×molcm/颗粒⋅s若不计外扩散阻力,则CAS=C==CCAGAAGG=1.68==1.681.68×10--55molmol/cmmmol/cmol/cm/cm3由教材www.hackshp.cn312页:*2φs=−(RALDeC)/AG2φφ=φηL=dp/6=0.04cm,可算得s=0.1375,由(6.82)式s,将此式与(6.60)式用试差法联立求解可得:φ=0.387η=0.927.多相催化反应器的设计与分析7.1若气体通过固定床的线速度按空床计算为0.2m/s,0.2m/0.2m/s,s,则真正的线速度为多少?已知所填充的固体颗粒的堆密度为1.2g/cm1.2g/cm1.2g/cm3,颗粒密度为1.81.8g/cmg/cmg/cm3解:ρb1.2ε=−1=−1=0.33330.30.3333333ρ1.81.8pu空床0.20.2u===0.6/0.0.6/6/ms真正ε0.33330.3330.333337.2为了测定形状不规则的合成氨用铁催化剂的形状系数,将其填充在内径为98mm的容器中,填充高度为1m,然后边续地以流量为1m3//hh的空气通过床层,相应测得床层的压力降为101.3Pa,实验操作温度为298K298K,,试计算该催化剂颗粒的形状系数.已知催化剂颗粒的等体积相当直径为4mm,4mm,4mm,堆密度为1.45g/cm1.45g/cm1.45g/cm3,颗粒密khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
度为2.62.6g/cmg/cmg/cm3.解:23∆p=101.101.33Pa=101.3kgmsLr/⋅,=1,mρ=1.185kgm/−5−5−3µ=1.87101.8711.8710×0Pas⋅=1.8710×kgmsd/⋅,=×410mv1u==0.03685/0.0360.03685/85/ms0236000.785(0.098)3600036000.785(0.098)×.785(0.098)ρ1.451.1.4545bε=−1=−1=0.44230.40.4423423ρ2.602.2.6060p2Lur0ρ(1−ε)由(7.1)式p=f∆3dεpkhdaw.com根据(6.4)式可推导出ψa=dp/dv,式中dp为等比外表面积相当直径,dv为等体积相当直径.Lu2ρ(1−ε)fpε3r0∆dv∴∆p=f即=(1)(1(1)(1))32ψadvεψaLur0ρ(1−ε)150115050µ(1−ε)由(7.2)课后答案网式f=+1.7515011.75150.75150=×+1.75(2)Reψduρav0将有关数值代入(1)和(2)式得:fwww.hackshp.cn−332=101.310101.3(410)(0.4423)/11.185(0.03685)1.3(410)(0.4423)/11.185(0.03685)(410)(0.4423)/11.185(0.03685)××(10.4423)39.07(10.4(10.4423)39.07−423)39.07=(3)ψa−31.8311.8310(10.4423)×0(10.4423)−8.778f=150×+1.751.71.755=+1.75(4)−3ψ(410)0.036851.185(410)(410)0.036851.185××0.036851.185×ψaa(3),(4)式联立:8.778/ψ+1.75=39.07ψ,将此式变为:aa239.07ψ−1.75ψ−8.778088.7780.7780=aa解此方程得:ψ=0.49690.4960.49699a7.3由直径为3mm的多孔球形催化剂组成的等温固定床,在其中进行一级不-1可逆反应,基于催化剂颗粒体积计算的反应速率常数为0.8s,有效扩散系数为20.013cm/s,当床层高度为2m时,可达到所要求的转化率.为了减小床层的压力降,改用直径为6mm的球形催化剂,其余条件均不变,,流体在床层中流动均为层流,试计算:(1)(1)催化剂床层高度;(2)(2)(2)(2)((2)2)床层压力降减小的百分率.解(1)((1)1)求dpddpp为6mm6m6mmm的床层高度L2,已知数据:dp:dp1=3mm=0.3cm,dp=3mm=0.3c=3mm=0.3cm,dpm,dp2=0.6cm,L=0.6c=0.6cm,Lm,L1=2m,=2m,kp=0.8skpkp=0.8s=0.8s-1,De,De=0.013cm,D,De=0.013cme=0.013cm=0.013cm2/skhdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
xAf1FdxLVA0∫0ηRAη1r11A2===LVxAf1η2r2Fdx1A0∫A0ηR2AR1kp10.300.3.30.800.8.8ϕ==×=0.39220.3920.3922213De320.0130.0.013013求得η=0.920.0.92921R2kp10.60.8ϕ==×=0.78450.7840.7845523De320.0130.0.013013求得η=0.75600.756.756khdaw.com2η0.920.0.9292∴L=1L=×=22.43222.432.43m21η0.75600.756.7562(2)求床层压力降减小的百分率:22Lu1ρ0(1−ε)Lu2ρ0(1−ε)∆p=f,∆p=f1课后答案网13223dεdεp1p2假定床层的空隙率不变,则有:∆p1fLd11p2=www.hackshp.cn(1)∆pfLd222p1层流流动时:151501515001−εf==150150×µReduρp0f1∴=d/d(2(2)(2(2))p2p1f2(1),(2)式联立:22∆p1Ldd11p2p2L1⎛dp2⎞2⎛0.6/20.6/0.6/22⎞==⎜⎟=⎜⎟=3.22533.225.225∆p2Ldd2p1p1L2⎜⎝dp1⎟⎠2.430.3/22.4302.430.3/2⎝.3/2⎠床层压力降减少的百分率为:∆p−∆p3.22513.2253.2251−112==0.689968.99%0.6890.689968.99%968.99%=∆p3.22533.225.22517.4拟设计一多段间接换热式二氧化硫催化氧化反应器,每小时处理原料气35000m3(标准状况下),),组成为SSOO2:7.5%;O::7.5%;O:7.5%;O2:10.5%;N:10.5%;N2:82%.采用直径5m5mmm高10mm10m10mmm的圆柱形催化剂共80m3,取平均操作压力为0.1216Mpa,0.1216Mpa,0.1216Mpa,平均操作温度为733K,混合气体的粘度等于3.4×10--55Pa.sPa.s,Pa.s,,密度按空气计算.解:由(7.1)式khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
2Lur0ρ(1−ε)∆p=f3dεp根据题给条件有:7330.10130.0.10131013350003350005000××u=2730.12132730.2730.12131213=21.75Ams−1/0360036360000A上式中AA——床层截面积,m2.−1L=80/880/0/A=80Am6V60.785(0.005)60.7860.785(0.005)5(0.005)20.01p××−1d===6.005106.0056.00510×10mp2a20.785(0.005)20.7820.785(0.005)×5(0.005)+4.140.0050.01××p1/31/31/31/36V60.785(0.005)60.7860.785(0.005)5(0.005)20.01khdaw.com⎛p⎞⎡××⎤−3d=⎜⎟=⎢⎥=7.215107.2157.21510×10mv⎝π⎠⎣3.14233.142.142⎦ψ=d/d在题(7.1)中已推导出apv,因此有:−36.005106.0056.00510×10ψ==0.83230.8320.83233a−37.215107.2157.21510课后答案网×10查”无机化工反应工程””P10P10P1088图4-4-11得ε==0.45,0.45,混合气的物性数据按空气计算误差不大,733K下,ρ=0.4832kg/m=0.4832k=0.4832kg/mg/m3,μ=0.034厘泊==3.43.4×10--55Pa.sPa.s,Pa.s,,因此有:−5150www.hackshp.cnµ(1−ε)1503.410(10.45)11503.410(10.45)503.410(10.45)××−f=+1.751501.7511.75150=50×+1.75=+1.75−3−1Reduρ6.00510(21.756.006.00510(21.75510(21.75×A)0.4832×p0=0.044440.0440.0444444A+1.75−1−1280A×0.4832(21.750.40.4832(21.75832(21.75A)(10.45)−∴∆p=(0.04444(0.04(0.04444444A+1.75)=1.838−336.005106.0056.00510×10×0.45−3×A(0.04444(0.04(0.04444444A+1.75)Pa要求△P<4052PaP1.838×107A--33(0.0444A(0.0444A+1.75)(0.0444A(0.0444A+1.75)+1.75)试差求解床径:床层直径D床层截面积A等式右边的值(m)(m)(m2)(P(Pa)(Pa)a)5.322.0546785.422.8942395.4523.3240395.5023.753849所以床层直径应大于或等于5.45m,直径为5.45m所对应床层高度为:80L==3.43133.431.431m23.32223.323.327.5多段冷激式氨合成塔的进塔原料气组如下:组分NH3N2H2ArAArrCHCCHkhdaw.comH4若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
%2.0921.8266.002.457.63(1)(1)(1)(1)((1)1)计算氨分解基(或称无氨基)进塔原料气组成:(2)(2)(2)(2)((2)2)若进第一段的原料气温度为407℃,求第一段的绝热操作线方程,方程中的组成分别用氨的转化率及氨含量来表示.反应气体的平均热容按33.0833.08J/molKJ/molKJ/mJ/molKolK计算.反应热△Hr=-5358J/molNHHr=-5358J/Hr=-5358J/molNHmolNH3(3)(3)(3)(3)((3)3)计算出口氨含量为10%时的床层出口温度,按考虑反应过程总摩尔数变化与忽略反应过程总摩尔数变化两种情况分别计算,并比较计算结果.解:(1):(1)计算氨分解基气体组成:100mol原料气中含NH32.09mol,2.09m2.09mol,2.09mol,相当于2.09/2molN2.09/2m2.09/2molNolN2及(1.5(1.5×2.09)2.09)molH2.09)m2.09)molHmolHolH2,因此,无氨基气体组成如下:NH3N2H2CHCCHH4ArAArr∑MoMMooMolMMololMolMMololMolMMololMoMMooMolMMololMoMMooMolMMololMolMMololMolMMololMolMMololMolMMololkhdaw.coml%%%l%l%%22.8667.77.62.4102.0069.147.637.4742.410052351(2)绝热操作线方程:(A)(A)(A)(A)(A)考虑反应过程中气体总摩尔数的变化.13N+H⇌NH课后答案网223以y代表氨基气体mol%,Fmol%,Ft表示混合气体总摩尔流量,由22可以看出,每生成1摩尔NH3,混合气体总摩尔数减少1,所以生成氨的摩尔数Fy−Fy=tAtwww.hackshp.cn0A0,(下标A代表NH3,0代表进口处,yA0和yA均指有氨基的mmol%)mol%)因此有:Ft0−(FytA−Fyt0A0)=Ft1+yA0化简得:F=F(1)(1(1))tt01+yA(a)(a)(a)(a)以N2的转化率表示组成时的绝热操作线方程:FN2,0∆xN2(−∆HrT0)=FCTP∆T上式中(-(-△HHr)Hr)以反应每kmolkmol的N2计.1+yA0∴FN2,0∆xN2(−∆HrT0)=FCt0p∆T1+yA∆T=FN2,0,,00(−∆HrT0)1+yA∆xFC1+yN2t0pA0以进口处N2的转化率为0作基准计算,则有:T=T+yN2,0(−∆Hr⎛⎜)1+yA⎞⎟x0C⎝1+y⎠N2pA0−∆Hr=(2525358253581×35811kJkmolN)/2代入有关数据:khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
0.21825358120.2180.21825358122535812××1+yT=680680+Ax33.0810.0209+N2T=680+692.4(1692.692.4(14(1+y)x化简得:AN2(2)又,生成的NH3molmmolol数为:1+yyF−yF=yFA0−yFAtA00t0At0A0t01+yA1⎛1+y⎞A0⎜yF−yF⎟At0A0t02⎝1+y⎠反应消耗N2的mol数为:A01⎛1+y⎞A0F⎜y−y⎟t0AA02⎝1+y⎠Ax=khdaw.comN20.2180.0.218221822F∴N2的转化率:t01⎛10.020910.010.0209+209⎞⎜y−0.02090.0200.02099⎟A2⎝1+y⎠Ax=N20.2180.0.218221822代入数据:x+0.047880.0470.0478888课后答案网y=N2A2.29122.291.291−x化简得:N2(3)⎛x+0.047880.0470.0478888⎞www.hackshp.cnT=680692.468069680692.4+2.4x⎜1+N2⎟N2⎜2.29122.291.291−x⎟(3)(3)代入((2)2)式得:⎝N2⎠x化简后得到以N2表示组成的绝热方程为:162016162020xT=680680+N22.29122.291.291−xN2(b)(b)(b)(b)(b)以NH3含量表示组成的绝热操作线方程:(FytA−Fyt0A0)(−∆Hr=)FCtp∆T式中(-(-△HHr)Hr)以每生成1kmolNH1k1kmolNHmolNH3计,⎛1+y⎞⎜FA0y−Fy⎟(−∆H=)FC∆TtAt0A0rtp⎝1+y⎠A(−∆Hr1)+yA⎛1+yA0⎞(−∆Hr⎛)yA0(1+yA⎞)∴∆T=⎜y−y⎟=⎜y−⎟AA0AC1+y⎝1+y⎠C⎝1+y⎠pA0ApA0−∆H=535815353581581kJkmolNH/r353581⎡0.0209(10.0200.0209(19(1+y)⎤A代入数据:T=680+y−33.08⎣⎢A10.020910.0210.0209+09⎥⎦化简得到以yA表示组成的绝热操作线方程如下:khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
T=6801620[+y−0.02047(1+y)]AA(B)(B)(B)(B)(B)忽略反应过程中气体总摩尔数的变化(a)以N2转化率表示组成时的绝热操作线方程:F(−∆H)∆x=FC∆TN2,0rN2tp式中-△Hr以每反应1km1kmolN1km1kmolNolN2计.∴∆T=FN2,0,0(−∆Hr∆)x=FN2,0,,00(−∆Hr∆)xFCN2FCN2tpt0pyN2,0(−∆Hr)0.21822535810.2180.21822535812253581××即T=T+∆x=680680+x0CN233.08333.083.08N2p化简之T=680+707.1xN2khdaw.com(b)以NH3含量表示组成的绝热操作线方程:Fyt(A−yA0−)(∆Hr=FCt)p∆T式中-△Hr以每反应1km1kmolN1km1kmolNolN2计:−∆Hr∆T=(yA−yA0)C课后答案网p−∆H=53581kJmolNH/r3535815535813581T=680+(yA−yA0=)T68016206806801620+1620(yA−0.0209)www.hackshp.cn33.08333.083.08(3)计算出口氨含量为10%的床层出口温度,考虑反应总摩尔数变化时:T=6801620[+y−0.02047(1+y)]6801620[0.10.02047(10)]6801620[0.10.02047(10.1)]=+−+.1)].1.1)])]AA=805.58805.505.5K忽略反应总摩尔变化时:T=6801620(+y−0.0209)=6801620(0.10.0209)+−=808.1KA808.1-805.5=2.6K,温度相差并不大,这是由于合成氨反应体系总转化率不高的缘故,若转化率高则两种方法计算出来的床层出口温度将会有较大的差别.7.6在绝热催化反应器中进行二氧化硫氧化反应,入口温度为420℃,入口气体中SO2浓度为7%(7%(mol);7%(7%(mol);mol);出口温度为590℃,出口气体中SO2含量为2.1%(2.1%(mol)2.1%(m2.1%(mol),mol)ol),在催化剂床层内A,B,CA,B,C三点进行测定.(1)(1)(1)(1)((1)1)测得A点的温度为620℃,你认为正确吗?为什么?(2)(2)(2)(2)((2)2)测得B点的转化率为80%,你认为正确吗?为什么?((3)(3)(3)3)(3)((3)3)测得C点的转化率为50%,经再三检验结果正确无误,估计一下C点的温度.解(1)绝热床内的温度是呈线性上升的,出口处温度最高,床内任一点温度不可能高于出口温度,故620℃是不可能的.(2)出口处SOSSOO2的转化率为((0.07-0.021)(0.07-0.021)×100%/0.07=70%.100%/0.07=70%.100%/0.07=70%.床层内部任一点处转化率不可能高于70%,故转化率为80%是不可能的.(3)(3)△tt==λ△XA,590-420=λ×0.7λ=(590-420)/0.7=242.86故C点温度为:t=t0+λ△XA=420+242.86=420+242.86×0.5=541.4℃khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
7.7乙炔水合生产丙酮的反应式为:2CH+3HO→CHCOCH+CO+2H2223322在ZnO-FeZnO-FZnO-Fee2O3催化剂上乙炔水合反应的速率方程为:73r=7.0610exp(7413/)7.0617.0610exp(7413/)×0exp(7413/)−TCkmolm/床层⋅hAA式中CA为乙炔的浓度,拟在绝热固定床反应器中处理含量为3%C2H2(mol)(m(mol)ol)的气体1000m3(STP)/h,(STP)(STP)/h,/h,要求乙炔转化68%,若入口气体温度为380℃,假定扩散影响可忽略,试计算所需催化剂量.反应热效应为-178kJ/mol,-178kJ/mo-178kJ/mol,l,气体的平均恒压热容按36.4J/molK36.4J/m36.4J/molKolK计算.解:原料气中乙炔浓度很低,可忽略反应过程总摩尔数的变化.F(1(1−x)(1−x)273)27)2733A0AAC==FAA0QQ0(273273+t)式中khdaw.comQ0为以标准状态计的体积流量,Q为温度t时垢体积流量.77273(1−xA)r=7.0610exp(7413/)7.0617.0610exp(7413/)×0exp(7413/)−TC=7.0610exp(7413/)×−TFAAA0Q(273((273273+t)00.680.0.68681xAfQ0(273273+tdx)AV=Fdx=FrA0∫0rAA0∫0F(1−x2737.0610exp(7413/)27372737.0610exp(7413/))×.0610exp(7413/)×7−T课后答案网AA0A0.6400.0.64.6464(273273+tdx)A=Q0∫07.061017.0617.06101×017(−x273exp(7413/))−Twww.hackshp.cnA5已知:−∆H=178kJmol/=1.7810×Jmol/C=36.4/JmolK⋅p5y(−∆H)0.0.031.7810031×.7810×λ=A0r==146.51146.546.5KC36.43636.4.4p绝热操作线方程为:t=t+λ(x−x)380146.5)380)380146.5=+146.5x0AA0A273273+t令:f(xA)=77.061017.0617.06101×01(−xA273exp(7413/))−T算出一系列f(Xf(XA)-X)-XA的值如下表:XA00.10.20.330.40.50.60.7t℃380395409424438452468482F(XF(XA)×2.862.522.282.092.022.012.12.39103图解积分得:0.680.0.6868−3−33∫0fxdx(A)A=1.5101.511.510×0所以Vr=10001.510110001.5100001.510××=1.5m7.8题7.7所述乙炔水合反应,在绝热条件下进行,并利用反应后的气体预热原料,其流程如图77AA所示.所用预热器换热面积50m2,乙炔浓度为3%的原料气以1000m3(STP)/(STP)(STP)//h的流量首先进入预热器预热,使其温度从khdaw.com100℃升到某一定值后进若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
入体积为1m3的催化剂床层中绝热反应,反应速率方程见题7.7,预热器总传质系数为17.5w/m17.5w/m17.5w/m2K,反应气体热容按36.4J/m36.4J/molK36.4J/m36.4J/molKolK计算,试求:(1)(1)(1)(1)((1)1)绝热温升(可不考虑反应过程中反应气体总摩尔数的变化).))..(2)((2)(2)(2)2)(2)((2)2)计算反应器出口可能达到的乙炔转化率(列出方程式,并用文字说明求解过程).解:(1)::(1):(1)绝热温升.按题意,在计算绝热温升时可忽略反应过程总摩尔数的变化.5y=0.03,−∆H=1.7810×JmolC/,=36.4/JmolK⋅A0rp5y(−∆H)0.0.031.7810031×.7810×λ=A0r==146.51146.546.5KC36.43636.4.4p(2)列方程求解转化率:(A)(A)由绝热床热量衡算得:Tf=T==TT0+146.5X++146.5X146.5XAfAAff(1)((1)1)khdaw.com(B(B)(B))由预热器热量衡算得::TT0-373=Tf-T--TT2(2)((2)2)(C(C)(C)(C))(C)(C)预热器中,传热的对数平均温度差为:(T2−373373−()Tf−T0)∆T=mT−373373ln2T−Tf0传热速率方程课后答案网:FCTtp(f−T2=UAT)∆m53而F=PQRTP/,=1.1.01.01310013101310×PaT,=273,KQ=1000m/,htwww.hackshp.cn33=R8.314108.3148.31410×10Pam⋅/kmolK⋅51.013101.0131.01310×10×1000−2F==44.63kmolh/=1.2410×kmols/t38.314108.3148.31410×10×273222又U=17.5/wm⋅K=17.5/Jsm⋅⋅Α=50Km17.55017.5517.550×0∆Tm(T2−T0−)(Tf−373373733733)T−T==1.938∆T=1.938(3)f2×−2××3mT−T1.21.2411036.410336.4106.410ln20T−373733733f(D)绝热床反应体积:xAf1V=FdxrA0∫A0kCA211221123221123++−−11233CA0(1−xTA0)δ==−0.5y=0.030.0.0303C=AA0A21+yδxTA0AA式中CA0为床层进口处浓度,而CAA00=P==PPA0AA00/R/RT/RTT0=0.03×1.013×105/(8.314/(8.314×103T0)=0.36655/T=0.36655/T0kmol/mkkmol/mmol/m3khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
0.36550.3650.36555(1−xTA0)3C=kmolm/AT0(10.01510.0110.015−5xTA)F=0.0344.671.340.0340.0344.671.34×4.671.34=kmolh/A0故有:xAfdxV=1.34A=1(4)1(1(4)4)r∫07(1−xA)T00.36550.0.365536557.0610exp7.0617.0610exp×0exp(−7413/T)10.01510.0110.015−5xTTA0联立解方程(1)-(4)((1)-(4)1)-(4)便可解出T0,Tf,T2,XAf7.9某合氨厂采用二段间接换热式绝热反应器在常压下进行如下反应:CO+HO→CO+H222热效应△khdaw.comHr=-41030J/mol,Hr=-41030J/Hr=-41030J/mol,mol,进入预热器的半水煤气与水蒸汽之摩尔比1:1.4,而半水煤气组成(干基)为:组成COH2COCCOO2N2CHCCHH4其他∑mmol%mol%30.437.89.4621.30.790.25100图7b为流程示意图,图上给定了部分操作条件,假定各股气体的热容均可按33.51J/molK33.51J/m33.51J/molKolK计算,试求Ⅱ段绝热床层的进出口温度和一氧化碳转化率,设系统对环境的热损失为零课后答案网.解:(1):(1)预热器热量衡算:FCt+FCt′=FCT+FC×300303003000p0p2pfp�105+www.hackshp.cnt2′=250300252503000300+=t2′445C(2)第一段绝热床热量衡算:t1′−t1=λ∆xA=λ(x1−x0)yA0(−∆Hr)0.3041410300.3040.304141030×141030λ===155.21155.255.2C11.433.4911.4311.433.49+3.49p�x−x=0.8−t′t=155.20.8124.115155.20.8124.15.20.8124.1×=C1011(3)由Ⅰ,Ⅱ段绝热床的中间换热器热量衡算得:FC300303003000+FCt′=FCt+FCtpp1p1p2t′−t=t−3003001112�上面已算出t′−t=124.1C,所以,t−300124.1,=故有:112�t=124.1300424.1124.1124.1300424.1+300424.1=C2(4)列第二段绝热床热量衡算:t2′=t2+λ(x2−x1−)t2′2t2=λ(x2−x1)把ttx,,′的值代入上式:445424.1155.2(:445:445424.1155.2(−424.1155.2(=x−0.8)22212解得:x=0.93470.930.93474727.10在氧化铝催化剂上进行乙腈的合成反应:khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
CH+NH→CHCN+H∆H=−92.292.292.2kJmol/22332r设原料气的摩尔比为C2H2:NH:NH3:H2=1:2.2:1,==1:=1:2.2:1,1:2.2:1,2.2:1,采用三段绝热式反应器,段间间接冷却,使每段出口温度均为550℃,而每段入口温度亦相同,已知反应速率式可近似地表示为:4rA=k(1−xA)kmolCH22/kgh⋅=k3.3.083.0810exp(7960/)0810exp(7960/)×10exp(7960/)−T式中xA为乙炔的转化率,液体的平均热容为Cp=128/JmolK⋅,如要求乙炔转化率达到92%,并且日产乙腈20吨,问需催化剂量多少?解:以A表示乙炔,32010×201022010010×F===22.09kmolh/A024×M×0.920.90.92224410.92××乙腈Ckhdaw.com在热衡算中忽略反应过程总摩尔数的变化,并把各段的p视为相等,对每一段均有:FCtp∆T=−(∆HFr)A0∆xA(−∆HFr)A0(−∆HrF)A0(−∆Hry)A0则有:∆T=∆x=∆x=∆xAAAFCCFCtppt0p−∆H课后答案网=92.2kJmol/=9.2210×4Jmol/r419.22109.229.2210×10∆T=∆x=171.51171.571.5∆xAA12.21www.hackshp.cn12.21++128依题意,各段进出口温度相等即各段△T相等,所以各段转化率差△XA亦相等,因此有:△XA=1/3=1/3×0.92=0.3067各段△T为:△T=171.5T=171.5△XA=171.5×0.3067=0.3067=52.59K52.59K因而各段进口温度==823-52.59=770.4K=823-=823-52.59=770.4K823-52.59=770.4K52.59=770.4K各段进出口温度和转化率如下表所列:进口出口段数T(K)T(T(K)K)XAT(K)T(T(K)K)XA一770.408230.3067二770.40.30678230.6134三770.40.61348230.92第一段T=770.4+171.5T=T=770.4+171.5770.4+171.5△XAk=3.08k=3.08×104exp(-7960/T)exp(-7960/exp(-7960/T)T)0.306710.30671w=Fdx=22.0922222.092.09.09dx1A0∫0A∫0ArAk(1−xA)XA00.050.100.150.200.250.300.3067T770.4779787.6796804.7813.3821.9823k1.0031.1241.2571.401.5581.7291.9151.9411/k(1-1/k1/k(1-(1-0.9970.93650.88390.840.80320.77110.74590.7431XA)图解积分求得:khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
0.3067dx∫A=0.25760.2570.257660k(1(1−x)A因此,w1=22.09×0.2576=0.2576=5.6905.690KgKKgg第二段,T=770.4+171.5(X,T,T=770.4+171.5(X=770.4+171.5(XA-0.3067)XA0.30670.350.400.450.500.550.600.613T770.4777.8786.8794.9803.6812.1820.7823k1.0031.1071.2381.3781.5371.7051.8591.9411/k(1-1/k1/k(1-X(1-XA)1.4381.3891.2471.3181.31010.3031.3231.3330.61341∫0.30670.3060.30677dxA=0.42120.4210.42122k(1(1−x)A故有:w2=22.09==22.0922.09×0.4212=0.4212=9.3049.304KgKKgg第三段,T=770.4+171.5(X,T,T=770.4+171.5(X=770.4+171.5(XA-0.6134)khdaw.comXA0.60340.650.700.750.800.850.900.92T770.4776.7785.2793.8802.4810.9819.5823k1.0031.0911.2191.3601.5141.6801.8651.9411/k(1-1/k1/k(1-X(1-XA)2.5792.6192.7352.9413.3033.9685.3626.4400.921∫0.61340.6130.61344dxA=1.041.1.0404k(1(1−x)课后答案网Aw3=22.09×1.04=1.04=22.9622.96KgKKgg催化剂总重量www.hackshp.cn=5.69+9.304+22.96=37.95==5.69+9.304+22.96=37.955.69+9.304+22.96=37.95Kg7.11例7.3所述的两段绝热式水煤气变换反应器,若第一段出口一氧化碳的转化率为84%,为使该段的催化剂用量最少,则第一段进口气体的温度应为多少?试利用题7.3所给的数据计算并与该题给定的第一段入口温度值相比较.解:用T0表示第一段入口温度,第一段绝热操作线方程为:T=TT=T0+155.2XA(1)((1)1)例7.3中((G)(G)式:⎡⎛1⎞⎤⎢∂⎜⎟⎥⎢⎜⎝(−RA⎟⎠)⎥65421654265421.674(1.674.674β−1)=⎢∂T⎥kp*x2T2⎢⎥A0(1−A1)(−β)⎢⎥⎣⎦xA*−4k=2.1722.172.17210exp210exp×10exp(−6542/Tmolg)/⋅min⋅Pa例7.3中(B)(B)式:将(B)(B)式代入(G)(G)式得:⎡⎛1⎞⎤⎢∂⎜⎟⎥⎢⎜⎝(−RA⎟⎠)⎥65426542165421.674(1.674.674β−1)=(2)(2)⎢∂T⎥−4TPx2T2⎢⎥2.17210exp2.1722.17210exp×10exp(−6542/A0)1−(A1−β)()⎢⎥⎣⎦xA将(1)(1)式代入((2)2)式得:khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
⎡⎛1⎞⎤⎢∂⎜⎟⎥⎢⎜⎝(−RA⎟⎠)⎥⎢∂T⎥⎢⎥⎢⎥⎣⎦xA65421654265421.674(1.674.674β−1)=(3)(3)−4222.17210exp2.1722.17210exp×10exp⎡⎣−6542/(T0+155.2xA)⎤⎦PA01(−xA1−)(βT0+)(155.2xA)而PA0=03.1267P==03.1267P03.1267Pt=0.1267==0.12670.1267×101325=101325=1.2841.284×104PPaPaa代入((3)3)式得:⎡⎛1⎞⎤⎢∂⎜⎟⎥⎢khdaw.com⎜⎝(−RA⎟⎠)⎥⎢∂T⎥⎢⎥⎢⎣⎥⎦xA65421.674654265421.65421.674(1.674674β−1)=−4422⎛−6542⎞2.17210×课后答案网×1.2841011.2841.284101×101(−xA)(1−βT)(0+155.2xAexp⎜)⎟⎝T+155.2x⎠0A31.6741.674β−1=2.346102.34610×(4)www.hackshp.cn22⎛−6542⎞(1−xA)(1−βT)(0+155.2xAexpexexpp⎜)⎟⎝T+155.2x⎠0A为使第一段催化剂用量最小,需符合(7.30)所表示的最佳化条件:⎛1⎞∂⎜⎟xAi+1⎜⎝RA(xTA,⎟⎠)[]dx=0;i=1,2,11,2,,2,⋅⋅⋅N(7.30)∫AxA∂TxA(4)式代入(7.30)式得:3⎛−6542665654254242⎞2.346101.6742.3462.346101.674×101.674(β−1exp)⎜⎟0.840.0.8484⎝T+155.21155.255.2x⎠0Adx=0∫0(1−x1)(−β2T)(+155.2155155.2.2x)2AA0A⎛−654265654242⎞(1.674β−1exp)⎜⎟⎝T+155.21155.255.2x⎠0A令fx(A)=2(5)(5)(1−xA1)(−βT0)(+155.2155155.2.2xA)上式变为:0.840.0.848432.3462.34610×10∫0fx(Adx)A=00.840.0.8484即∫fx(Adx)A=0(6)(6(6))0khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
符合(6)式的T0即为所求的最佳第一段入口温度.上面有关式子中的β,可用例7.3中((C)(C)式求得:3444(3.993.9913.99110110×10+1.28310×xA)(1.59410×+1.28310×xA)β=4441.2831011.2831.283101×101(−xA(5.90910)×−1.28310×xA)KpKp=0.0165exp(4408/)0.0160.0165exp(4408/)5exp(4408/)T例7.3中(A)式将(1)式代入例7.3(A7.3(A)7.3(A)7.3(A))式得:Kp=0.0165exp[4408/(TKp=0.0165expKp=0.0165exp[4408/(T[4408/(T0+155.2XA)]))]](7)((7)7)将((7)7)式代入例7.3中(C)((C)(C)式得:3444(3.993.9913.99110110×10+1.28310×xA)(1.59410×+1.28310×xA)β=444⎛440844440808⎞khdaw.com1.2831011.2831.283101×101(−xA(5.90910)×−1.28310×xA)×0.165exp⎜⎟⎝T+155.21155.255.2x⎠0A(0.394+0.1267xA)(0.15750.12760.1570.15750.127650.1276+xA)=478.3(8)⎛440844440808⎞(1−xA0.58330.12670.580.58330.1267)(330.1267−xA)exp⎜⎟⎝T+155.21155.255.2x⎠0A用(8)式计算β,(5)式计算课后答案网f(XA),通过试差便可求得符合(6)式的T0,设T0=670K,得到不同XA值的β和f(XA)值如下表:XAβf(XA)www.hackshp.cn07.068×10-3-0.03886--0.038860.038860.20.02357-0.02893--0.028930.028930.40.06812-0.02893--0.028930.028930.60.1970-0.02358--0.023580.023580.70.35330.023920.80.70240.059480.840.978447.29作f(XA)~XA图,过f(XA)=0处划一水平线,以此线为界,线下的阴影面积代表:xop∫0fx(Adx)A其值为负线以上的阴影面积代表:0.840.0.8484∫fx(Adx)dxA其值为正xop两块阴影面积几乎相等,说明0.840.0.8484∫0fx(Adx)A=0符合(6)式,因此所设T0=670K(397℃)即为所求.<讨论>与例7.3的计算结果相比较.例7.3—在规定的一段入口温度(663K)和最终转化率(91.8%)下,各段温度和转化率的最佳分配是:一段出口转化率为85%,二段入口温度为663K.但这样的一段入口温度(633K)并非最佳,因它不能保证第一段符合最佳化条件,(7.30)式.若要使第一段符合(7.30)式,则一段入口khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
温度就不是633K.本题---在规定的一段出口转化率(84%)下,一段出口温度的最佳值是670K,这样的分配符合(7.30)式.第二段的入口温度可用最佳化条件(7.30)式,若要第二段也符合(7.30)式,则最终转化率就不是91.8%.7.12图7.C和图7.D分别为两个化学反应的T-X图,图中AB为平衡曲线,NP为最佳温度曲线,AM为等温线,GD为绝热线,GK为非绝热变温操作线,HB为等转化率线.khdaw.com(1)试比较这两个图的差异,并说明造成这些差异的根本原因.(2)采用固定床反应器进行图7.C所示反应,分别按MA,GD和GK操作线操作,要求最终转化率达到50%,试比较这三种操作所需催化剂量的大小,说明原课后答案网因.(3)对图7.D所示的反应,重复(2)的比较.(4)对于(2)和(3)的比较结果,你认为是普遍规律呢还是个别情况.www.hackshp.cn解:(1):图7.C图7.DA.T升高,平衡转化率减小T升高,平衡转化率增大B.有最佳温度曲线无最佳温度曲线C.绝热操作线斜率为正绝热操作线斜率为负D.非绝热变温操作线有热点非绝热变温操作线有”冷点”造成以上差异的根本原因是:图7.C是可逆放热反应的X-T关系,而图7.D是可逆吸热反应的X-T关系.(2)因是可逆放热反应,操作线接近TOP线的程度越大,催化剂用量越小,从图7.C看,在转化率从0到50%这一范围内,MA线最接近TOP曲线,所以等温操作所需催化剂最少,绝热操作(GD线)居中,非绝热变温操作(GK线)催化剂用量最大.(3)对图7.D,7.D7.D,,是吸热反应,反应温度高则催化剂用量小,从图7.7.DD看,G,GKK线的操作温度最高,催化剂用最最小,绝热操作居中,等温操作温度最低,因而催化剂用量最大.(4)等温操作线的位置(即等温操作所维持的温度)对(2),(3)(2)(2),(3),(3)的比较结果有很大影响,例如图7.C的等温操作线MA左移(即降低等温操作的操作温度),它与TOOPP曲线的接近程度就会发生变化,与GDGD线和DDKK线相比,在转化率0到50%范围内,M,MAA线不一定最接近TOOPP线,因而不一定是等温操作所需催化剂用量最小.对图7.D,如果等温操作线MMAA右移,即提高等温操作的温度,可使MA,GDMAMA,GD,GD和GK各线的操作温度的高低顺序发生变化.另外,如果最终转化率不是50%,例如是70%,对图7.C,在反应后期(即转化率接近70%的部分)最接近TOP线的是GGDD线,绝热操khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
作的催化剂用量最小(反应后期接近TOOPP线的程度对催化剂用量大小起关键作用.所以说,(2),(3),(2),(2),(3),(3)比较结果,并非普遍规律.7.13在一列管式固定床反应器中进行邻二甲苯氧化制苯酐反应,管内充填高及直径均为5mm的园柱形五氧化二钒催化剂,壳方以熔盐作冷却剂,熔盐温度为370℃,该反应的动力学方程为:0r=0.04170.0410.04177ppexp(13636/)−Tkmolkgh/⋅sAB0p式中PA为邻二甲苯的分压PPa,a,B为O2的初始分压PPa,a,反应热效应△HHr=-1285kHr=-1285kJ/mol,r=-1285kJ/mol,J/mol,反应管内径为25mm,25mm,25mm,原料气以9200kg/m9200kg/m9200kg/m2h的流速进入床层,其中邻二甲苯为0.9%,空气为99.1%(mol),99.1%(99.1%(mol),mol),混合气平均分子量子力为29.45,平均热容为1.072kJ/kgK,1.072k1.072kJ/kgK,J/kgK,床层入口温度为370℃,床层堆密度为1300kg/m1300kg/m1300kg/m3,床层操作压力为0.1013Mpa(0.1013Mpa(0.1013Mpa(绝对),总传热系数为69.8w69.8w/m69.8w/m69.8w/m2K,试按拟均相一维活塞流模型计算床层轴向温度khdaw.com分布,并求最终转化率为73.5%时的床层高.计算时可忽略副反应的影响.解:以A表示邻二甲苯,对邻二甲苯作物料衡算得:−dF=−ρrAdlAbs式中rS为邻二甲苯的反应速率,因邻二甲苯含量很小,系统系统总摩尔数可看作成恒定不变,Ft是常数且等于FtFt0,Ft0,0,故有:−dFdy=ρrAdlt0课后答案网AbsFdpt0A因y=p//p,则有:−=ρrAAtbspAdlwww.hackshp.cnt1FMFMdpdpt0mA即−=ρrMbsmpAdltFMt0m而=GAdpAMmρbMmρb0∴−=prts=pt×0.040170.0400.0401717ppAEexp(−13636/T1)()dlGG已知数据:325M=29.429.455ρ=1300kgmG/=9200kgm/⋅hp=1.01310×Pambt054p=0.9910.211.013100.9910.9910.211.01310×0.211.01310××=2.10810×PaE将已知数据代入(1)式得:dpA29.4529.429.45130051300×130054−=×1.013101.0131.01310×10×0.04017pA×2.10810exp×(−1363/T)dl9200929200008=3.5703.57010×10pAexp(−13636/TPam)/()2式(2)便是物料衡算方程,式中PA的单位是PPa.a.热量衡算方程:按(7.10)式khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
dT4UGCp=ηρ0bsr(−∆Hr−)(T−Tc)dldtdTρb(−∆Hr)4U若η0=1,1,则有:=rs−(T−Tc)3()dlGCdGCpttpt已知数据:TC=643K,==643K,=643K,643K,dt=0.025m,dt=0.025m,ddt=0.025m,t=0.025m,△Hr=-1285kJ/mol=-1.285Hr=-1285kHr=-1285kJ/mol=-1.285J/mol=-1.285×106kJ/mol,kkJ/mol,J/mol,C2﹒K=2.513K=2.513×102kJ/mkJ/m2﹒h﹒K.K.代入((3)3)式有:pt=1.072k=1.072kJ/kg==1.072kJ/kg1.072kJ/kgJ/kg﹒KU=69.8w/mKU=69.8w/KU=69.8w/mm6dT13001.285101300113001.28510×.28510×4=×0.040172.108100.0400.040172.10810172.10810××pAexp(−13636/T)dl92001.0719200192001.071×.071242.5131042.5142.51310×310×−(T−643643)0.02592001.0710.0250.02592001.071×92001.071×3khdaw.com=1.436101.4361.43610×10pAexp(−13636/T−4.080)T−643(Km/4)()(4)式中PA的单位是Pa,式(2),(4)((2)(2),(4)2),(4),(4)是一常微分方程组,可采用数值解法,初值条件为:l=0;l=0;T=643K;T=T=643K;643K;PA=yA0AA00PtPt=0.009PtPt=0.009=0.009×1.013×105=911.7Pa=91=911.7Pa1.7Pa计算结果如下:邻二甲苯转化率邻二甲苯分压PA床层温度床层高度lxA(Pa)(Pa)(K)(K)(m)(m)课后答案网(%)911.764300827.5663.60.39.233700.9www.hackshp.cn678.90.623.12556.96880.938.91430.3686.11.252.28344.9674.91.562.17295.4663.41.868.6241.66572.073.57.14试分析下列说法是否正确:(1)在一绝热反应器中进行无热效应的零级反应,其转化率与反应器长度的关系是线性的.(2)在一绝热反应器中仅当进行一级反应时,其反应温度与转化率的关系才呈线性.(3)(3)(3)(3)((3)3)多段绝热反应器最优化的结果是各段的催化剂相等.解::(1):(1)从(7.9)式得:dx/dZ=ρMyr/GwAbA0AA0绝热条件下的无热效应反应,床层各处等温,而且由于是零级反应,rA与CA无关,所以对于不同的Z,rA为常数,若忽略内,外扩散的影响,η0=1,则dXddXXA/dZ=//d/dZ=dZ=Z=常数,即XA与Z呈线性关系.若系统存在扩散影响,则还需考虑CA对η0的影响问题.(2)不对,对绝热反应,有如下热量衡算式:dTGCpt=ηρ0bAr(−∆Hr7.21)()dZ固定床反应器的物料衡算式为:khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
GwdxA0A=ηρr(7.97.9)0bAMdZA两式相除得:dTwA0(−∆Hr)=(7.227.7.2222)dxMCAAptwA0(−∆H)T−T0=(xA−xA0)(7.2377.7.23.2323)MC积分之Apt若将(-(-△HHr)Hr)视为常数则(7.23)式表明T与XA呈线性关系,从上面推导过程可以看出(7.23)式不受一级反应的限制,非一级反应一样可以使用.(3)((3)3)无此结论.khdaw.com7.15常压下用直径为6mm的球形氧化铝为催化剂进行乙腈合成反应,操作条件与习题7.10同,此时内扩散影响不能忽略,而外扩散影响可不计,氧化铝的物理性质如下:孔容0.45cm3/g,//g,g,颗粒密度1.1g/1.1g/cm1.1g/cm1.1g/cm3,比表面积180m2/g,曲节因子等于3.2.试计算第一段的催化剂用量.解:r’A----------------反应速率,kmol/m,k,kmol/mmol/m3粒子﹒hρb------------颗粒密度,kg/m,kg/m3粒子r’43A=ρb×3.08×10exp(-7960/T)(1-Xexp(-7960/exp(-7960/T)(1-XT)(1-XA)kmol/hmkmol/hkmol/hmm粒子,而r’A=kpC=k=kpCpCA=kpC=k=kpC课后答案网pCA0(1-(1-X(1-XXA)T0/T,//T,/T,kp是以颗粒体积计的反应速率常数,因此,4ρp(1−xA×)33.083.0810exp.0810exp×10exp(−7960/T)k=pwww.hackshp.cnCA0(1−xTTA0)/4ρp(1−xA×)33.083.0810exp.0810exp×10exp(−7960/T)=pTA0000(1−xA)RTT0RT4−1=ρp×3.0810exp3.0813.0810exp×0exp(−7960/Th)pA031ρ=1100kgM/y=p=101325110132501325PapA0t12.21112.21+2.21+33R=8.314108.3148.31410×10Pam⋅/kmolK⋅代入数据得:38.318.3148.31410410×104−1kp=1100T×3.0810exp3.3.0810exp0810exp×(−7960/Ts)1×10132510132101325512.21112.21+2.21+化简之7−13−1kp=1.161.1671.16710710×10Texp(−7960/Th)=3.24210×Texp(−7960/Ts)题给:Vg=0.45=0.45cmcmccmm3/g//ggSg=180m==180m180m2/g=180//g=180/g=180×104cm2/g//gg平均孔径<=2V<=2Vra>=2V4==55×10--77g/Sg=2==22×0.45/0.45/180180×10cmccmm常压下气体分子运动的平均自由程近似等于10-5khdaw.comcmcm,cm,,因此,λ/2=10/2=/2=1010-5/(/(2/(22若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
×5×10-7)=)=10,)=10,10,可见以努森扩散为主,乙炔分子量为26,故:−7−42D=9700510970059700510××10T/26=9.51110×Tcm/skε=Vρ=0.4510.451.10.495×.10.495=τ=3.2pgpmεp0.49500.495.495−4−42De=D=×9.511109.5119.51110×10T=1.47110×Tcm/skτm3.23.2Rk0.33.242100.33.0.33.2421024210×3Texp(−3980/393980/80/T)p1/41/1/41/44φ===469.5Texp(−3980/T)−41/41/1/443De31.471101.41.471107110×T由7.10题解知,第一段绝热热线方程是T=770.4+171.5xT=T=770.4+171.5x770.4+171.5xA1/41/4φ=469.5770.4469.5469.5770.4(770.4)exp−3980/770.4()=14.11进口处khdaw.com出口处φ=469.5770.4171.50.3067exp469.5770.4171.469.5770.4171.50.3067exp(+50.3067exp×)⎡⎣−3980/770.4171.503980/770.4171.50.3067(+×.3067)⎤⎦=19.96可见,第一段床层各处内扩散影响严重,因此有η0=1/φ第一段催化剂用量:0.30670.3060.3067710.30671w=Fdx=Fdx1A0∫AA0∫A0ηr010Ar课后答案网Aφ1/1/41/440.30670.3060.30677469.546469.59.5Texp(−3980/T)=FdxA0∫03.0810exp3.0813.0810exp×0exp4(−7960/T1−)(xA)www.hackshp.cnA1/41/40.30670.3060.306770.01520.0150.01522Texp3980/(T)=dx∫0(1−x)AA1/41/40.01520.0150.01522Texp3980/(T)0.300.0.303067令fx(A)=则w1=FA0∫fx(Adx)A(1−x)0A计算得到XA与f(Xf(XA)的一系列数值如下:XA00.050.100.150.200.250.300.3067T(K)T(T(K)K)770.4779787.6796804.7813.3821.9823F(XF(XA)h﹒14.0313.9914.0114.1014.2314.4414.7414.79kg/kmolkkg/kmolg/kmol图解积分求得:0.30670.3060.30677∫0fx(Adx)A=4.311⋅hkgkmol/(7.10)题已算出FA0=22.09==22.09=22.09kmol/h,22.09kmkmol/h,ol/h,因此有:w1=22.09×4.314.311=95.234.311=95.234.311=95.23Kg7.16在三段绝热式固定床反应器中进行n级不可逆反应,各段的催化剂量相同,且控制进入各段的反应物料温度相等.若n>0,n>0,试问(1)(1)(1)(1)((1)1)哪一段的净转化率最大?哪一段最小?khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
(2)(2)(2)(2)若段间采用冷激方法进行降温,试问第一段与第二段之间和第二段与第三段之间哪处需加入的冷激剂量多?为什么?(3)(3)(3)(3)((3)3)若n<0,nn<0,n<0,对问题(1),(2)(1)(1),(2),(2)是否还能作出肯定性的回答?为什么?解:(1):(1)虽然各段进口温度相等,在催化剂量相同的情况下,净转化率也按1至3段的顺序递减,第一段最大,第二段次之,第三段最小.(2)因第一段反应量大于第二段反应量,所以1-2段间要取出的热量比2-2-33段间要取出的热量大,因而1-2段间需要的冷激量也大于2-32-2-33段间需要的冷激剂量.((3)3)若n<0,nn<0,n<0,则反应速率按1至3段的顺序递增,第一段净转化率最小,第三段最大.1-2段间的冷激剂量小于2-32-2-33段间的冷激剂量.khdaw.com7.17在内径为5.1c5.1cmm的固定床反应器中常压下进行萘氧化反应:采用直径为0.318cm的球形钒催化剂,该反应可按拟一级反应处理,以床层体积为基准的反13−1k=5.7415.5.7410exp7410exp×0exp(−19000/Ts)应速率常数为:反应热效应△Hr=-1796kJ/mHr=-1796kHr=-1796kJ/mJ/m2K,萘在钒催化剂内的有效扩散系数等于1.2×10-3cmccmm2/s,//s,/s,若床层的热点温度为641K,试计算热点处气相中萘的浓度.假定外扩散的影响可不考虑,副反应可忽略课后答案网.解:球形粒子Rkpφ=3www.hackshp.cnDe1313135.5.7415.74107410×05.7410×kp=k/1(−ε=)exp(−19000/119000/9000/T=)exp(−19000/T)1−ε10.4110.4−0.413−1=9.56610exp9.5669.56610exp×10exp(−19000/Ts)1311330.3189.566100.3180.3189.566109.56610×7φ=exp(−9500/959500/00/T=1.49610exp)×(−9500/T)−323×1.2101.21.210×10热点处Tmax=641K==641K641K7φ=1.49610exp(9500/641)5.476(3)1.4961.49610exp(9500/641)5.476(3)×10exp(9500/641)5.476(3)−=>11∴=η==0.1820.0.182618266φ5.47655.476.476外扩散影响可忽略,因此η0=η=0.1923热量衡算式:dT4UGCpt=η(−RA)(−∆Hr−)(T−Tc)dZdt式中(-RA)是以床层体积为基准的反应速率.热点处dT/dZ=0,因此有:4Uη0(−RA)(−∆Hr=)(Tmaxmamaxx−Tc)dtkhdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
所以,热点处的反应速率为:4UTmaxmax−Tc41418064180641630×80641630(41630−)3−(RA=)=−2=0.47350.4730.47355molm/⋅sdtη0(−∆Hr)5.110××0.18267901000(×)−43=4.735104.7354.73510×10kmolm/⋅s−而(RA=)kCA则CA=−(RA/)k1313−1热点处k=5.7410exp5.7415.7410exp×0exp(−19000/641)=7.689s因此,热点处CA的值为:−4(−RA)4.734.7354.73510510×10−33C===6.159106.1596.15910×10kmolm/Ak7.68977.689.689khdaw.com7.18习题7.17中的萘催化氧化反应器的原料气为萘与空气的混合气,其平均定压热容为1.3kJ/KgK,若进入床层的原料气温度与熔盐温度相等.催化剂的最高耐热温度为700K,试问:(1)(1)允许最高的熔盐温度是多少?(2)(2)(2)(2)((2)2)如选定熔盐温度为640K,那么床层进口气体中萘的最高允许浓度是多少?解:(1)因是一级反应,且进床层原料气温度与冷却介质温度相等,故可用课后答案网(7.62)式:RT14RT4R2Rmaxmax≤[1[[11−1−c]可改写为1−T=−1T()1cmaxmaxEwww.hackshp.cn2EEE已知E/R=19000Tmax=700K,代入(1)式得:4T2C1−=−1×7001900019000解得:Tc=674K所以最高熔盐温度为674K(2)最大时料浓度由(7.63)式求得:λNN≤+1C+C(7.6377.63.63)T−TeemaxmaxC式中N=4/UρCdkCpttc(−∆Hwr)A0λ==−(∆HCr)A0/ρCptMCApt已知数据:TC=640K==640K640KTmax=700KTmTmax=700Kax=700KU=180w/mUU=180w/m=180w/m2Kρ=PM/RT=101325=PM/R=PM/RT=101325T=101325×29/(8.31429/(8.31429/(8.314×103×640)=0.5522640)=0.5522640)=0.5522Kg/mKg/m3Cpt=1.3==1.3=1.3kJ/KgK1.3kJ/kJ/KgKKgKdt=5.1×10-2--22mKC是按冷却介质温度计算的反应速率常数,因此KC=5.74==5.745.74×1013eexp(-19000/exp(-19000/640)=7.341exp(-19000/640)=7.341sxp(-19000/640)=7.341640)=7.341s-1khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
4180414180×80∴N==2.67922.679.679C3−20.55220.3100.5520.55220.31020.310×××5.110××7.341将有关数据代入(7.63)式:λ2.6792.679=+1+70064070064700640−0ee解得:λ=178.9=178.9KKλρCpt178.178.9178.90.5522130090.55221300×0.55221300×3∴C===0.071510.0710.0715151molm/A03(−∆Hr)17961017961179610×033=7.15107.1517.1510×0kmolm/7.18在充填10m3催化剂的绝热固定床反应器中进行甲苯氢解反应以生产苯:CHCH+H→CH+CHkhdaw.com6532664原料气的摩尔组成为3.85%C6H6,3.18%C6H5CH4,69.97%H2;温度为863K,操作压力为6.08M6.08Mpa;6.08Mpa;6.08Mpa;若采用空速为1000m3(STP)/hm(STP)(STP)/hm/hm3催化剂,试计算反应器出口的气全组成.该反应的速率方程如下:30.5r=5.7310exp(17800/)5.7315.7310exp(17800/)×0exp(17800/)−TCCTTH式中CT和CH分别为甲苯和氢的浓度,kmol/m,k,kmol/mmol/m3,甲苯转化速率rT的单位为kmol/mkkmol/mmol/m3s,反应热效应课后答案网=-49974==-49974=-49974-49974J/mol,J/mol,J/mJ/mol,ol,为简化计,反应气体可按理想气体处理,平均定压热容为常数,等于4.23J/molK.4.23J/4.23J/molK.molK.解:p=6.08www.hackshp.cnMPa=R8.314108.8.3141031410×3Pam⋅3/kmolK⋅t6pT00.03186.08100.0310.03186.081086.0810××3C===0.026950.0260.0269595kmolm/T03RT8.314108.3148.31410×10×86306pH00.69976.08100.6990.69976.081076.0810××3C===0.59290.5920.59299kmolm/H03RT8.314108.3148.31410×10×8630C=C(1−xT)0=0.026958630.0260.0269586395863×(1−x=)23.2523.23.25251−xTTT0TTTTTTT1C=(C−Cx)0=(0.59290.026950.5920.59290.0269590.02695−x)0=(511.723.25−x)HH0T0TTTTTT60.0.50.0.55rT=5.7310exp5.7315.7310exp×0exp(−17800/TCCT)H61−x0.50.5T=5.7310exp5.7315.7310exp×0exp(−17800/T×23.25)1.51.5(511.723.25−xT)T81−x0.50.53T=1.332101.3321.33210×101.51.1.51.55(511.723.25−xT)exp−17800/(Tkmolm)/⋅sTkhdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
(∆Hry)T04949974499740.03189740.0318×0.0318又T=T+λx=863+x=863+x=886337.576337.57+x0TTTTC42.34242.3.3pt总摩尔流量F=100010/22.4446.410001100010/22.4446.4×0/22.4446.4=kmolh/=0.124kmols/t−3所以有:F=yF=0.03180.1240.030.03180.124180.124×=3.94310×kmols/T0T0txT1VrxT1V=Fdx∴=dx()1rT0∫0rTF∫0rTTT0TVr103==2536252253653636m⋅skmol/−3F3.943103.9433.94310×10T01.51.5xT1xTTexp17800/exp17exp17800/(800/T)3∫0dxT=∫080.50.5dxTm⋅skmol/()2khdaw.comrT1.1.3321.332101332101×101(−xT511.723.25)(−xT)()2式代入(1)式得:1.51.1.51.55xTTexp17800/exp17exp17800/(800/T)dx=253625253636∫080.50.5T1.3321011.3321.332101×101(−xT511.723.25)(−xT)1.5课后答案网Texp17800/exp17exp17800/(800/T)令fx(T)=80.0.50.551.3321011.3321.332101×101(−xT511.723.25)(−xT)xT则有:∫0www.hackshp.cnfx(Tdx)dxT=253622536536计算得到不同xT下的f(xf(xT)值如下表:xT00.10.050.20.250.30T863866.8868.6870.5872.4874.3F(xF(xT)763278227965812883298575从图解积分得知:xT∫0fx(Tdx)dxT=253625253636所以,反应器出口甲苯转化率为33%,反应器出口气体组成计算如下:(以100mo100m100mool原料气为基准)床层出口组分床层进口摩尔数床层出口摩尔数mol%mol%甲苯3.183.18(1-0.33)=2.1313.18(3.18(1-0.33)=2.1311-0.33)=2.1312.131%H269.9769.97-3.18×0.33=0.33=68.9268.9268.92%苯3.853.85+3.183.85+3.85+3.183.18×0.33=0.33=4.8994.8994.899%甲烷2323+3.1823+23+3.183.18×0.33=0.33=24.0524.0524.05%∑100100100%7.20充填新鲜催化剂的绝热床反应器当进口原料的温度控制为460℃时,出口物料温度为437℃,转化率符合要求;操作数月后,由于催化剂的活性下降,为了khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
保持所要求的转化率,将原料进口温度提高到470℃,出口物料温度相应升至448℃;若反应的活化能为83.7kJ/mol,83.7k83.7kJ/mol,J/mol,试估计催化剂活性下降的百分率.xAf1v=FdxrA0∫A0kfx(A)解:式中f(xf(xA)只是与动力学方程式中的浓度项有关,而与催化剂活性无关,在催化剂活性下降后,,f(xf(xf(xA)是不变的,但k值减小了(对相同温度而言),为了在Vr不变的情况下保持要求达到的转化率,就需要提高操作温度,在较高的操作温度下,活性必低后的催化剂仍能维持原来的k值,从而保持所要求的转化率.阿累尼乌斯方程式lnk=lnA-E/RT,lnk=llnk=lnA-E/RT,nA-E/RT,以下标1代表催化剂活性好时,下标2代表活性下降后,假定催化剂失活其活化能不发生改变.活性好时:lnklnk1=lnA=l=lnAnA1-E/RT--E/RTE/RT1活性下降后:lnklnk2=lnA=l=lnAnA2-E/RT--E/RTE/RT2床层处处都应存在如下关系khdaw.com:lnk:l:lnknk1=lnk=l=lnknk2,则有:EElnllnnA−=lnA−12RTRT12AE⎛11⎞lnllnn1=⎜−⎟ARTT也可写成2⎝12⎠由△T=课后答案网λ△xA关系可知,对于某一△xA值,活性降低前与降低后的△T相等的,取△T的不同数值计算A1/A2,结果如下:在△T=-5KT=T=-5K-5K处,A1www.hackshp.cn8383700700⎛11⎞ln=⎜−⎟=0.18740.180.187474A8.31427346058.3148.3142734605⎝2734605+−2734705+−⎠2AA/=1.2061.201.206612在△T=-10KT=T=-10K-10K处,A18383700700⎛11⎞ln=⎜−⎟=0.18990.180.189999A8.314273460108.3148.31427346010⎝27346010+−27347010+−⎠2AA/=1.2091.201.209912床层出口处A18383700700⎛11⎞ln=⎜−⎟=0.21630.210.216363A8.3142734608.3148.314273460⎝273460+273470+⎠2AA/=1.2421.241.242212以上计算结果表明,在床层的不同高度(即不同的xA处),A1/A),A),A1/A21/A2的值并不相等,即催化剂活性下降的百分数不相等,但差别不大,床层进出口处活性下降的百分数为:A−A1.24211.2421.2421−112×100%=×10100%19.42%0%19.42%=A1.241.1.242412A→P+Q7.21在实验室中用外循环式无梯度反应器研究二级气相反应khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
原料为纯A;设kCkkCCA0AA00τ==1.1.试计算A的转化率,当:(1)(1)(1)(1)((1)1)循环比为5;(2)((2)(2)(2)2)(2)((2)2)循环比为30;(3)((3)(3)(3)3)(3)((3)3)按全混流模型处理;(4)((4)(4)(4)4)(4)((4)4)比较上列各问题的计算结果并讨论之.解:是二级等摩尔反应,因此有:22−RA=kCA0(1−xA)循环反应器反应体积的计算式:xAf1Vr=(1−ψQC)0A0∫ψxAfdxA(4.244.44.24.2424)1+ψ(−RA)(4.24)式可写为:xAf1khdaw.comkCA0τ=(1+ψ∫ψ)ψxAf2dxA1+ψ(1−xA)其中τ=Vr/Q//QQ0,把kCkkCCA0AA00τ=1代入得:xAf11=(1+ψ∫ψ)xAf2dxA1+ψ(1−xA)由上式解得:课后答案网21+ψ(1+ψ)−=11−x1+ψψ−xAfwww.hackshp.cnAf(1)(1)当ψ=5时,215115+5(151+5)−=11−x11551155+−55xAfAf2化简得:5x−17x+=60AfAf解得:x=3(不合理舍去)x=0.400.4.4Af1Af2因此,A的转化率为40%(2)当ψ=30时,21301+30(130131301+300)−=11−x13030130313030+−0xAfAf2化简得:30x−929922x+310=AfAf解得:x=0.38530.3850.38533x=2.68(不合理舍去)Af1Af2得A的转化率为38.53%(3)全混流:khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
QC0(A0−CA)QCx0A0AfQCx0A0AfV===r2−R−RkC01−xAAA0(Af)VQ/=τr0xAf∴kCτ=A02(1−xAf)把kCA0AA00τ=1代入上式得:xAf1=2(1−xAf)2x−33x+=10则有:khdaw.comAfAfx=2.618(2.618(不合理舍去)x=0.382解得:Af1Af2所以,A的转化率为38.2%(4)当循环比为30时,计算得到的转化率与全混流的计算结果很相近,可见大循环比的循环反应器可达到全混流操作.7.22列管式催化氧化反应器所用的催化剂管的内径为课后答案网34mm,34mm,34mm,管内充填钒钼催化剂,床层高度1.6m,含量为1.2%(1.2%(mol)C1.2%(1.2%(mol)Cmol)C6H6的苯空乞混合气以0.4m/s0.4m/s0.4m/s的速度(按空床计算)送入催化剂床,温度为673K;管外用673K的恒温盐浴冷却;床层与熔盐间的传热系数等于www.hackshp.cn100w/m100w/m100w/m2K;;K;;操作压力为0.213MPa;0.213MPa;0.213MPa;有关苯氧化反应的模型及动力学数据见习题4.16.试计算床层出口的气体组成及顺丁烯二酸酐的收率.催化剂床层的堆密度为1500kg/m1500kg/m1500kg/m3,混合气的热容可近似按空气计算.解:用B代表苯,M代表顺酐,C代表CO和COCCOO2等.根据习题(4.16)的题给条件:r1−kpk1B,1=A1eexpxp(−E1/RT=0.2171exp)(−70800/8.314T)=0.2171exp0.2170.2171exp1exp(−8515.7/TkmolkghPa)/⋅⋅8r22−kp2M,k2=1.37210exp1.371.37210exp210exp×(−193000/8.314T)8=1.37210exp1.3721.37210exp×10exp(−23213.8/TkmolkghPa)/⋅⋅r−kpk,=470.8exp470.470.8exp8exp(−124800/8.314T)33B3=470.8exp470.8470.8expexp(−15010.8/TkmolkghPa)/⋅⋅上面各式中r1,r2,r3均以每小时,每Kg催化剂转化的苯的kkmolkmol数来表示,PB和PM的单位用Pa;PPa;a;以rM表示对每小时,每KKgg催化剂来说,转化为顺酐的苯的kmokmol数,rC表示对每小时,每Kg催化剂来说,转化CCOO和COCCOOkhdaw.com2的苯的kmkmolkmolol数,则有:若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
r=r−rr,=r+rM12C23又YM表示顺酐的收率,YC表示CO和COCCOO2的收率.(一)物料衡算方程:对顺酐作物料衡算rρdV=FdY,rρAdZ=FdYMbrB0MMbB0MdY111M=ρr=ρr=ρr()1bMbMbMdZFB0/AQC0B0uC0B0A已知5p=0.2030.0.203203MPa=2.0310×PaT=673Ky=0.012t0B033=R8.314108.3148.31410×10Pam⋅/kmolK⋅5pB0ypB0t0.0122.03100.0120.0122.0310×2.0310×−43∴C====4.354104.3544.35410×10kmolm/khdaw.comB03RTRT8.314108.3148.31410×10×673003u=0.4/ms=1440/11440/440/mhρ=1500kgm/0b代入(1)式得:dY150015150000M=r=2392.2392.44r()2−4MMdZ课后答案网14404.345101440414404.34510×.34510×同理,对CO,COCOCO,CO,CO2作物料衡算得:rρdV=FdYCbrB0CdY1C∴www.hackshp.cn=ρbCrdZuC0B0dYC代入数据得:=2392.232392.492.44rC()3dZ(1),(2),(3)式中,rM,rC的单位用kmol/kgh,Zkmol/kkmol/kgh,Zgh,Z的单位用m.原料气中苯的含量很小,可认为反应过程中总摩尔数不变,因此,5pB=pB0(1−YC−YM=yp)B0t1−YC(−YM=0.0.010.0122.031010122.0310122.03101×)×(−YC−YM)=246312246314631(−YC−YMPa)5p=pY=ypY=0.0122.03100.0120.0122.0310×2.0310×Y=2436YPaMB0MB0tMMMrM=r1−r2=2436k1(1−YC−YM−243622436436)kY2Mkmolkgh/⋅()4rC=r2+r3=2463kY2M+246322463463k3(1−YC−YMkmolkgh)/⋅()5⎧k1=0.2171exp0.2170.2171exp1exp(−8515.7/TkmolkghP)/⋅⋅⎪⎪8⎨k2=1.37210exp1.3721.37210exp×10exp(−23213.8/TkmolkghP)/⋅⋅()6⎪⎪⎩k3=470.8exp470.8470.8expexp(−15010.8/TkmolkghP)/⋅⋅(二)热量衡算方程khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
dT4UGCpt=ρbr1(−∆Hr1+)ρbr2−∆H(r2+ρbr3)−∆Hr3−((T−)TC)()7dZdt混合气体平均分子量:10011001.1001.2−.221001.2−M=×0.21320.2130.2132×2+×0.79280.0127829.43×+×=m100110000100又pQt0=FRTt00Mpumt0=(MFmt0RT)0QMp0mt=(GART)0Q0Mp5AmtuMp00.429.432.03100.4290.429.432.0310×.432.0310××22mt∴G===kgm/⋅=s1538kgm/⋅s3RTRT8.314108.3148.31410×10×67300222C=1.059kJkgK/⋅T=673KU=100/wm⋅K=3.610×kJhm/⋅⋅Kkhdaw.comptCd=34mm=0.0340.00.03434mt反应1,2,3的热效应分别为:(-△Hr)HHr)Hr)1=1850=1850kJ/molkJ/kJ/molmol(-△Hr)HHr)Hr)2=1340=1340kJ/molkJ/kJ/molmol(-△Hr)HHr)Hr)3=3169kJ/kJ/molkJ/kJ/molmol将有关数据代入(7)式:课后答案网dT3315381153815381.059×1.059.059=150018501000(×r1+1500134010)1500134010(×r2+1500316911500316910)×0r3(dZ243.61043.6143.610××0−www.hackshp.cn(T−673673)0.03400.034.034化简得dT666=1.071.0731.07310310×10r1+1.23410×r2+2.91810×r3−26(T−673)8()dZ(8)式中Z的单位用m,r[1],r[2],r[3]m,r[1],rm,r[1],r[2],r[3][2],r[3]的单位用kmol/kgh,kmol/kkmol/kgh,gh,根据题给的条件得:⎧r=24362436(2436(1k(11−Y−Y)kmolkgh/⋅11MC⎪⎨r2=243624243636kY2Mkmolkgh/⋅()9⎪⎩r=2436(12436(2436(1k1−Y−Y)kmolkgh/⋅33MC联立求解(2)(3)(4)(5)(6)(8)(9)(2)(3)((2)(3)(4)(5)(6)(8)(9)4)(5)(6)(8)(9)各方程,即可求出YM和YC及T沿床高Z的分布,微分方程的初值条件为:Z=0,T=673K:Z=0,T=:Z=0,T=673K:Z=0,T=673KY673KYM=0YC=0==00(1)((1)(1)(1)1)(1)((1)1)床层出口气体组成:找出Z=1.6mZZ=1.6mZ=1.6m处的YM和YC的值,若以100mol进口气体为基准,则出口气体中苯的mol数为1.2(1.2(1-Y1.2(1-Y1.2(1-YM-YC),)),,生成的顺酐mmolol数为1.2YM,生成CO,COCOCO,CO,CO2的mol数为6×1.2YM,由以上基本数据便可计算出口气体的组成.(2)Z=1.6((2)Z=1.62)Z=1.6处的YM,即为床层出口的顺丁烯二酸酐收率.8.多相反应器8.1纯二氧化碳与氢氧化钠水溶液进行反应,假定液相上方水蒸汽分压可不计,试按双膜模型绘出气相及液相中二氧化碳浓度分布的示意图khdaw.com.若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
解:8.2用1.21.2MM的氨水吸收某生产装置出口气中的二氧化喘,当气流主体中二氧化碳分压为khdaw.com1.013×10-3MpMpaMpaMpaa时,该处的二氧化碳吸收速率为多少?已知:液相中CO2和NHNNHH3的扩散系数均为3.5×10-5cmccmm2/s,//s,/s,二级反应速率常数为38.6×105cm3/mols,//mols,mols,二氧化碳的溶解度系数为1.53×-103Pa,k--101022310molmol/cmmmol/cmol/cm/cmL=0.04cm/s,k==0.04cm/s,k0.04cm/s,kG=3.22×10molmol/cmmmol/cmol/cm/cmsPa,sPa,相界面积aL=2.0cm=2.0cm/cm/cm.解:−33P=1.0131.1.0131001310×10MPa=1.01310×PaAG课后答案网−33C=1.2M=1.21.1.22moll/=1.210×molcm/B−52D=D=3.510×cm/sALBLwww.hackshp.cn53k=38.61038.6138.610×0cm/mols⋅2−1011003H=1.53101.5311.5310×0molcm/⋅PaA23k=0.04cms/,//,,a=2.0cm/cmLL−1011002k=3.22103.2213.2210×0molcm/⋅⋅sPaG按拟一级反应处理,反应速率常数:5−33−1k=kC=38.6138.638.610×100×1.210×=4.63210×s2B35八田数:M=kD/k=4.632104.64.632103210××3.510/0.0410.06×=ALLβ=M=10.06属于快速反应,其增大因子:因此:(−RA=)kG(pAG−pAG*=βkpL)AG*HA亦即:khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
−10103−1011003.3.2213.221022×100(1.01310×−p*)=10.060.04×p*×1.5310×AGAG3p*=5.23×(1.0131011.01310.01310×−p*)AGAG35.231.013105.2315.231.01310×.01310×p*==850850PaAG6.236.6.2323−10(−RA=)βkpLAG*HA=10.060.048501.531010.0610.060.048501.5310×0.048501.5310×××−82=5.233105.2335.23310×10molcm/⋅s8.3气体A与液体B的反应为不可逆反应,对A为一级,对B为零级,已知三种情况下反应速率常数k,液侧传质系数kL,组分A在液相中的扩散系数DAALL以及液相体积与液膜之比α值如下:5kkD×10LALkhdaw.comαs−1cms/2cm/s4000.0011.61.1.66404000.040.0.04041.61.1.664010.040.0.04041.61.1.6640试分别计算这三种情况下的增大因子和有效因子值,并对计算结果进行比较与讨论.课后答案网解:−5kD1AL14001.404001.61001.610×610×M===801www.hackshp.cnk0.0010.00.001.001001L1β=M=801111−4η===3.3.1253.1251012510×101αM4080404080×801−5kD2AL24001.6104001.4001.610×610×M===22k0.040.0.0404L22×(401−+)tah2ttah2ah2c()22390.964(×+)β=×2==2.0732.072.073322×(401tah2401t401tah2−ah2)c+()12390.9641××+M(α−1+)tachM2401(−+)tach2()η==2αM⎡⎣M(α−1tachM)+1⎤⎦4022×⎣⎡×(40140401−1tach)2+()1⎤⎦2390.9642390.2390.964×+964−2==1.295101.2951.29510×10802390.964180239802390.9641(××0.9641+)khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
kD−53AL31111.6111.610×.610×0M===0.10.0.11≪13k0.040.0.0404L3αM400.01400.0400.01×13β===0.28780.2870.287883αM−M+1400.010.011400.400.010.011×010.011−+3311η===0.7190.0.7194719443αM−M+11.3933上述三组数据,随M的变小,β也变小,表示化学反应速率依次变慢,故液相利用率η依次增加.8.4在机械拌釜中于0.891Mpa0.891Mpa0.891Mpa及155℃等温下用空气氧化环已烷.液相进料中环已烷浓度7.74kmol/m7.74k7.74kmol/mmol/m3,氧含量为零.液体与空气的进料量分别为26.76m3//hh和161mkhdaw.com3/h,/h,要求出口的液体中环已烷浓度为6.76kmol/m6.76k6.76kmol/mmol/m3,假定气相及液相均呈全混流,气相阻力可忽略不计,试计算所需的反应体积.已知数据:该反应对氧及环已烷均为一级,操作条件下的反应速率常数k=0.2mk=0.2m3/mols,//mols,mols,氧的溶解度=1.115×10-4--44kmkmol/mkkmol/mmol/mol/m3.液侧传质系数kL=0.416cm./s,=0.416cm./s=0.416cm./s,,比相界面积=6.75cm=6.75cm-1,气含率εG==0.139,0.139,氧在液相中扩散系数DALAALL=2.22==2.222.22×10--44cmccmm2/s.//s./s.解:题给出的反应器出口液体中环已烷浓度值6.76kmol/m6.76k6.76kmol/mmol/m3太小了,即使气体中的氧完全反应课后答案网,也不能使环已烷浓度低到6.76kmol/m6.76k6.76kmol/mmol/m3,为此假定反应器出口环已烷浓度为7.657.65kmol/mkmol/mkkmol/mmol/m3;�3−33P=00.891.891MPaΤ=155CC=7.7477.74.74kmolm/=7.7410×molcm/www.hackshp.cnB03333C=0molm/Q=26.726.26.77m/h=7.43310×cm/sA0L3333Q=161m/hC=7.6577.65.65kmolm/=7.6510×molcm/gB3k=0.416cms/=k0.2m/mols⋅L−43−73氧的溶解度=1.11511.1151.1151×10kmolm/=1.115101.11.115101510×molcm/-1−42比相界面积a=6.75cm气含率ε=0.139D=2.2210×cm/sGAL由于环已烷大量过剩,按拟一级反应处理:3−4kCDBAL0.210××7.652.2210××M===1.40011.4001.40011k0.41600.416.416L1k0.4160.410.4166Lα====277.62277.677.6−4aδaD6.752.22106.7526.752.2210×.2210×LAL由(8.15)式khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
M(α−1+)tachM1.421.4277.61(77.61−+0.8854)β=M=1.4×=1.51.58080M(α−1tachM)+11.4277.611.4271.4277.61(7.61−×0.88541)+−43−73C=1.1151.1.1151011510×10kmolm/=1.11510×molcm/AiQ30g161101161106110×F===7187.57187.7187.55molh/=1.9965mols/22.422.4222.42.40N:F=0.79F=1.5773mols/2g00O:F=0.21F=0.4193mols/2A代入(8.31)-(8.33)((8.31)-(8.33)8.31)-(8.33)式⎛0.2100.0.21.2121⎞−71.57731.5771.57733⎜−fALr⎟=0.4166.75××Vr×1.581.11510(×−CALr)khdaw.com⎝0.790.0.7979⎠−730.4166.750.4160.4166.75×6.75×Vr×1.581.11510(×−CALr)=7.43310×(CALr−0+)5−3Vr(10.13921010.1310.139210−9210×)×7.6510×CALr−3−3−35−37.433107.4337.43310×10(7.7410×−7.6510×)=1Vr(10.139210−×)×7.6510×CALr化简得:课后答案网⎧−=×−7−0.260.2650.2658588fALr2.8128Vr(1.11510CALr)⎪⎪−7−3−3⎨4.43664.4364.43666Vrwww.hackshp.cn(1.11510×−CALr)=7.43310×CALr+1.317310×VCrALr⎪−3⎪0.66890.6680.66899=2.634710×VC⎩rALr解之得:f=0.0540.0.054460544646ALr533V=6.761106.7616.76110×10cm=0.676mr−103−43C=3.7552103.7553.755210210×molcm/=3.75510×molm/ALr8.5采用直径为1m的鼓泡塔用空气氧化环已烷,压力为0.912Mp0.912Mpaa的空气自塔底送入,,其他条件同习题8.4,假定气体呈活塞流,液体呈全混流,试问塔高应为多少?已知数据:a=2.5cm:a=:a=2.5cm2.5cm-1;k;;kkL=0.12cm/s;==0.12cm/s;0.12cm/s;εG==0.12,==0.12,其他数据见习题8.4.解:在计算鼓泡塔时,需要知道溶解度常数,而题8.4是无此数据,在这里,查得常温时ρL=0.7785g/cm=0.7785g/=0.7785g/cmcm3,设反应条件下:ρL=0.65g/cm=0.65g/=0.65g/cmcm3;a=2.5cma=2.5cma=2.5cm-1;kL=0.12c=0.12cm/s=0.12cm/s;m/s;;εG==0.12==0.123−4kCDBAL0.210××7.652.2210××八田数M===4.857>3k0.120.0.1212Lβ=M(−RA=)βkCLAi=kCDCBALAikhdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
对气相作物料衡算:−Fdf=aACkCDdZ()1gAcAiBALfA其中:CAi=HpAAi=HpAAG=HpA(1−rZ)2()1+fA将(2)代入(1((1)1))式得:faAHpkCDAcABAL−dfA=(1−rZdZ)()31+fFAg(3)(3)式的边界条件:X=0f=fAA0Z=Lrf=fkhdaw.comAALr积分(3)(3(3))式得:⎡f⎤aAHpkCD⎛r⎞LArcABAL2−⎢f−f+ln⎥=⎜Lr−Lr⎟4()LArA0⎣课后答案网fA0⎦Fg⎝2⎠0.210.0.2121上式中F=1.57731.5771.57733mols/f==0.2658gA00.790.0.7979若进入液相的氧均参加反应www.hackshp.cn:2Fg(fA0−fALr=QC)B0−(CB)3−3−32121.5721.57730.2658×.57730.2658730.2658(−fALr)=7.433107.7410×(×−7.6510×)f=0.053740.0530.0537474ALrπ23.141593.1413.1415959232A=d=×100=7.854107.7.8541085410×cmct443ρm=ρt(1−εG=)00.650.6510.12.6510.1210.12−(=0.572/)gcm60.5720.101325100.5720.5720.10132510×0.10132510×5−1r==6.1484106.1486.148410410×cm61033.60.912101033.1033.60.9121060.91210××代入(4)式得:360.050.0530.05374374742.57.85410×××0.91210×H−[0.053740.2658ln[0.05[0.053740.2658ln3740.2658ln−+]=A0.26580.2650.265881.5773−55−3−4⎛6.1484106.1486.148410410×2⎞210××7.56102.22107.7.56102.221056102.2210××⎜Lr−Lr⎟⎝2⎠化简,得:khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
−59⎛6.14866.148410.148410410×2⎞1.81066.166101.8101.81066.1661066.16610=×H⎜Lr−Lr⎟A⎝2⎠−59−13⎛6.1484106.1486.148410410×2⎞1.81066.166101.8101.81066.1661066.16610=××6.844610×⎜Lr−Lr⎟⎝2⎠解之得:Lr=395::Lr=395cm=3.95Lr=395cm=3.95cmcm=3.95m=3.95mLr3.95==3.953.3.9595d1t故处于3~12之间.8.6在充填直径为4mm的Pd-AlPPd-Ald-Al2O3催化剂的滴流床反应器中,于0.1013Mpa,500.1013Mpa,500.1013Mpa,50℃等温下进行α-甲基苯乙烯加氢反应,液相进料α-甲基苯乙烯的浓度为khdaw.com4.3×10-4molmol/cmmmol/cmol/cm/cm3;床层入口处纯氢及液体的线速度分别等于12cm/s12cm12cm/s/s和1cm/s;1c1cm/s;m/s;若液相中α-甲基苯乙烯转化率为90%;试计算床高,假定气相和液相均呈活塞流,且床层压力降可忽略不计.已知数据:该反应对氢为一级,对α-甲基苯乙烯为零级,操作条件下,基于床层体积计算的数据如下:k=16.8:k:k=16.8=16.8s-1;k;;kkLaL=0.203s==0.203s0.203s-1--11;k;;kkLSLLSSaS=0.203s==0.203s0.203s-1--11;氢在催化剂中的有效扩散系数=1.02×10-6--66cmccmm2/s;//s;/s;氢在液相中的溶解度系数=0.6cm=0.6cm3气/cm/cm3液;床层孔隙率ε=0.42.解:课后答案网�−43d=4mm=p0.1000.1013.101313MpaT=50CC=4.310×molcm/pB0−1u=12cms/u=1cms/x=0.900.9.9=k16.8sg0www.hackshp.cnL0Lf−1ka=0.203s=ε0.42LL−1−6233ka=0.203sDe=1.0210×cm/sH=0.6cm气/cm液Lg反应器入口处氢的浓度:5p1.011.0131.01310310×103−53C===37.70537.7037.7055molm/=3.770510×molcm/A0RT8.314273508.3148.31427350(27350+)当苯乙烯转化率达到90%时,氢的转化率xgL:uCx=uCxg0A0gLl0B0BL(计量系数1:1)−5−4123.770510123.7123.770510×70510×x=×14.310××0.9x=85.53%gfgf由于所给动学参数以床层为基准,故以颗粒为基准的k−1k==16.8/10.4216.8/16.8/10.4210.42−=28.97sp1−εRkp0.20.2−6φ==28.97/1.021028.9728.97/1.0210/1.0210×=355.33De31−3η==2.2.8142.814610814610610×φ由于反应只与氢有关,且为一级反应,因此有:khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
duCx(0gA0g)=KCOGAG()1dZ本题中,由于是纯氢,CAG=C==CCA0AA00,dxgu0g=KOG()2dZ从氢的溶解度系数的单位可知,(8.40)式−11⎛111⎞1⎛111⎞K=⎜++⎟=⎜++⎟OG−3Hka⎝kakη⎠0.60.2030.60.0.60.203⎝2030.20316.82.814610××⎠Lllss=51.67551.671.67s−2−1K=1.935101.9351.93510×10sOGkhdaw.com代入(2)式dxg−212=1.935101.9351.93510×10dZ12dZ=dx−2g1.1.9351.93510课后答案网93510×1012120.8553120120.8553×.8553Z=x==530cm=5.35.5.33m−2gf−21.935101.9351.93510×101.93510×8.7在反应体积为5m2的机械搅拌釜中,采用直径为0.9mm的催化剂于25℃等温下进行亚油酸甲酯的加氢反应,氢的分压保持为www.hackshp.cn0.81Mpa0.81Mpa0.81Mpa;亚油酸甲酯的处理量等于1.5kmol/min,1.5kmol/m1.5kmol/min,in,要求酯的40%被加氢,试根据例8.5所给的数据和计算结果,计算催化剂用量。解:Vr=5m3dp=0.9mmddp=0.9mmp=0.9mmT=298.15KT=298.15KpH=0.81Mpa=0.81M=0.81MpapaQ0=1.5kmol/min=1.5kmol/=1.5kmol/min=1.5kmol/minxminxAf=40%==40%40%*3(−RA=)Qx0Af/Vr=1.50.4/5×=0.12kmol/min⋅m假设此时液膜阻力可忽略,由(8.51)式**⎛11⎞(−RA=)CA⎜+⎟()1⎝WkasLssWksη⎠参考例8.5可知*0007.3C=×08100187.=.kmolm/AL0303.−05.又由于k∝d,故09.mm催化剂的Lsp05.−6175010⎛×⎞k=⎜⎟=01521.m//minminLs−360910⎝.×⎠662a===3333.m/kgs−33dρ0910.×××210ppkhdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
23k=m/kg⋅minmminin309.φ=×3=36.075.1η==02778.φ代入(1)式:−1⎛11⎞01200187.=.⎜+⎟⎝W×015213333.×.W×0277823.×/⎠ss012.⎛11⎞3W=⎜+⎟=4731.kgm/skhdaw.com00187015213333.⎝.×.0277823.×/⎠8.8苯胺催化加氢反应对氢为一级,对苯为零级;1.01Mpa1.01Mpa1.01Mpa和130℃下,采用直径为4mm的催化剂和纯氢进行反应,k=51.5cmk=k=51.5cm51.5cm3/gs,k/gs,kLs=0.008c=0.008cm/s=0.008cm/s,km/s,k,kLaL=0.12s-1--11,纯氢的溶解度3.56×10-6molmol/cmmmol/cmol/cm/cm3,氢在催化剂上的有效扩散系数为8.35×10-6cmccmm2/s,//s,/s,试求宏观反应速率。3/gs,/gs,kk解:课后答案网P=1.01MpaPP=1.01MpaT=130=1.01MpaT=130T=130℃dp=4mmddp=4mmp=4mmk=51.5cmk=k=51.5cm51.5cmLs=0.008c=0.008cm/s==0.008cm/s0.008cm/sm/skLaL=0.12s-1--11*−63−62C=3510.×molcm/Q=83510.×cm/sAL0本题中缺少一个重要参数,即颗粒密度,设ρwww.hackshp.cnp=2g/cm=2g/=2g/cmcm3;24πR366−1−1a=====15cmk=kρ=5152103.×=sspp43Rd04.πRp3Rkp02.103−3φ===234=η4270910.×−63De383510.×−1**⎛111⎞(−RA=C)AL⎜⎜+⎟⎟kakakη⎝LLLssp⎠−1−6⎛111⎞=35610.×⎜++⎟−3⎝012.0008151034270910.××.×⎠−73=1879610.×molcm/⋅s8.9在浆态反应器中用纯氢将丁炔二醇加氢为丁烯二醇,该反应对氢及丁炔均为一级;进反应器的丁炔二醇溶液浓度为2.5kmol/m2.5k2.5kmol/mmol/m3;反应条件下,k=4.8k=4.8×-53/kg/k/kg/kgg﹒s﹒mol,kmol,k-1--113;10mLaL’=0.3s,kLsLLss=0.005c=0.005cm/s=0.005cm/s,m/s,,氢在液相中的溶解度=0.01kmol/m=0.01kmol/=0.01kmol/mm液相中催化剂含量为0.1kg/m0.1kg/m0.1kg/m3,ρ0=1.5g/cm=1.5g/=1.5g/cmcm3,d,d’’s=40ms=40m2/kg,/kg,气液均呈活塞流。(1)试计算液体转化率为95%时过程各步阻力占总阻力的百分率,设内扩散阻力可以忽略。(2)计算液相转化率达95%时的空速khdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
(3)在最终转化率保持不变的情况下,提出提高液空速所采取的措施。3-53/k/kg/kgg﹒s﹒mol,mol,k-1--11解:CB0=2.5m=2.5mol/m==2.5mol/m2.5mol/mol/mk=4.8k=4.8×10mLaL’=0.3s,kLsLLss=0.005c=0.005cm/s=0.005cm/s=0.005m/s=0.005=0.005×10-2m/m/sm/ssWs=0.1kg/m=0.1k=0.1kg/mg/m3a’s=40m2/kg//kg/kg*3333C=001.kmolm/=10molm/ρ=15.gcm/=1500kgm/ALp(1)(1)(1)(1)此时CBLBBLL=C==CCB0BB00(1-1-0.951-0.950.95)==0.050.05×2.5=2.5=0.125kmol/2.5=0.125kmol/m0.125kmol/mm3=125mol/m==125mol/m125mol/m3各步阻力:11==3333.s液膜阻力kaLL′03.11==5000s液固阻力WaksL′Ls0140000005.××.11==1667s−5khdaw.com反应阻力WkCsBL014810.×.××125各步阻力所占的分率:气膜3.333/(3.333+5000+1667)=0.053.333/(3.333+5000+1667)3.333/(3.333+5000+1667)=0.05=0.05%液固5000/(3.333+5000+1667)=74.96%5000/(3.333+5000+1667)5000/(3.333+5000+1667)=74.96%=74.96%反应1667/(3.333+5000+1667)=24.99%1667/(3.333+5000+1667)1667/(3.333+5000+1667)=24.99%=24.99%−1课后答案网**⎡111⎤()(()2)−R=C⎢++⎥AAL⎣kaLL′WaksL′LsWkCsB0(1−xB⎦)www.hackshp.cnxBfdxBxBfCB0⎡111⎤τ=C=⎢++⎥dxB0∫0−R*∫0CkaWakWkC1−xB(A)AL*⎣LL′sL′LssB0(B⎦)2500095.⎡8335.⎤=⎢33335000.++⎥dx10∫0⎣1−x⎦BB2500⎡0.95⎤=⎣50030958335×.−.ln(1−xB0)⎦102500=[47532497+.]=1250000=s3472.h1011−1S===000288.hvτ3472.(3)提高反应温度,增加溶液中催化剂的含量。8.10今有含SO2的空气需要净化处理,采用以活性碳为催化剂,以水为液体介质的滴流床反应器在0.101Mpa0.101Mpa0.101Mpa,25℃下将SO2氧化为SOSSOO3,溶于水而成稀硫酸从反应器底部流出,反应的控制步骤是氧在催化剂表面的吸附,反应速率可用下式表示:3r=ηρkCmolcm/床层⋅sApA0式中rA为以O2表示的反应速率,CAA00为催化剂表面处的khdaw.comO2浓度,单位为mol/cmmmol/cmol/cm3;若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
已知:内扩散有效因子η=0.6,堆密度ρb==1.0g/cm=1.0g/=1.0g/cm1.0g/cmcm3,一级反应速率常数3/g﹒s,ss,,床层孔隙率ε==0.30.3,k-1--11-1--11k=0.06cmk=k=0.06cm0.06cmLaLLaaas=0.3s==0.3s0.3s,kLaL=0.03s==0.03s0.03s,气体的流量=100cm=100cm3/s,O/s,O2在水中溶解度的享利常数H=5.0H=5.0;反应器直径10cm,塔顶入口处气体组成SSOO22%,O219%,N279%,试求SOSSOO2的转化率为80%时滴流床反应器的床层高度。解:P=0.101Mpa:P=0.101Mp:P=0.101MpaaT=25T=25℃η=0.6ρ3k=0.06cmk=k=0.06cm0.06cm3/g﹒sb=1.0g/=1.0g/cm=1.0g/=1.0g/cmcmε==0.30.3kLaLLaaas=0.3s==0.3s0.3s-1--11kLaL=0.03s==0.03s0.03s-1--11Qg=100c=100cm=100cmm3/sH=5.0H=H=5.0H=5.05.0000d=10cmy=2%y=19%y=79%x=80%tSO2O2N2Af整个反应的宏观动力学方程:(−RA=)KCOGAG其中−1⎛111⎞K=H⎜++⎟khdaw.comOGA⎝kaLskaLssηρbk⎠⎛111⎞=50.⎜++⎟=3222.⎝003.030.0610006.×.×.⎠−3−1K=3103710.×sOG6p课后答案网p010110.×3G===4075.molm/RT831429815.×.03C=Gy=40752.×%=08149.molm/A0www.hackshp.cnSO2CA0(1−xA)C=AG1+δyxAA0A1对于SO+O→SO2232115−.δ==−05.A1CA0(1−xA)1−xAC==CAGA0105002−.×.x1001−.xAAkhdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com
π2π22Ac=dt=(10)=7854.cm44xAfdxQ0xAf1001−.xAL=QCA=Adxcr0A0∫∫A0KCK01−xOGAGOGA=Q0xAf10011+.(−xA−)001.=Q0⎡−−+⎤Lr∫0dxA⎣099.lnllnn(1xA)001.xAf⎦AK1−xAKcOGAcOG100=[−099.ln.0200108+.×.]−378543103710.×.×=6569.cm=657.m8.11在以甲苯为液相介质,骨架镍为催化剂的浆式反应器中,进行乙烯加氢反应,反应条件:P=1.52MpaPP=1.52Mpa=1.52Mpa,T=50T=50℃,进料C2H4/H2=1/1.5==1/1.5=1/1.5(摩尔比),H2的进料速率为khdaw.com1.386mol/s,1.386m1.386mol/s,ol/s,已知αL=1.5cm=1.5cm2/cm/cm3,kL=0.015cm/s==0.015cm/s0.015cm/s,αS=200m2/kg//kg/kg,kLs=0.02c=0.02cm/s==0.02cm/s0.02cm/sm/s,Ws=0.1kg/m=0.1k=0.1kg/mg/m3,H2在甲苯中溶解的享利常数为9.4,液相可视为全混流;反应过程的速率决定于氢从气液相界面处向液固相界面间的传递,试求乙烯转化率为50%时所需的反应体积。0F=1.386mols/解:P=1.52MpaPP=1.52Mpa=1.52MpaT=50T=50℃C2H4/H2=1/1.5==1/1.5=1/1.5H2aL=1.5cm=1.5cm课后答案网2/cm/cm3kL=0.015cm/s==0.015cm/s0.015cm/sas=2000cm=2000cm2/gkLsLLss=0.02c=0.02cm/s==0.02cm/s0.02cm/sm/sH=9.4H=9.4Ws=0.1kg/m=0.1k=0.1kg/mg/m3=0.1×10--33g/cg/cmg/cmm3乙烯转化率为www.hackshp.cn50%时,设氢转化率为xH;1×50%=1.5xH解得:xH==0.33330.3333设反应的宏观动力学*(−RA=KC)OGAG−1⎛11⎞⎛11⎞KOG=HA⎜+⎟=94.⎜+−3⎟⎝kaWak⎠⎝150015.×.0110.××2000002×.⎠LLssLs3=276710.×s−4−1K=361310.×sOGxAfdxxAfdxV=FA=FArA0∫A0∫0(−RA)0KCOGAG0F=F=1386.mols/A0H26p15210.×3C===56576.molm/AGRT8314323.×.15V=FA0x=138603333.×.=226.m3rAf−4KC361310.××56576.OGAGkhdaw.com若侵犯了您的版权利益,敬请来信通知我们!℡www.khdaw.com'
您可能关注的文档
- 传感器与检测技术 (徐科军 著) 电子工业出版社 课后答案
- 化学反应工程 第二版 (陈甘棠 著) 化学工业出版社 课后答案
- 2016化学与人类文明课后习题答案
- 化学反应工程 第二版 (郭锴 著) 化学工业出版社 课后答案
- 线性代数(居余马 著)第二版 清华大学出版社
- 6000考研俄语高频词汇 课后答案
- 高等数学同济第六版下册课后答案
- 传感器原理及应用 (王化祥 张淑英 著) 天津大学出版社 课后答案
- 向对象程序设计c++ (李爱华 程磊 著) 清华大学出版社 课后答案
- 传感器原理及应用 (张洪润 张亚凡 邓洪敏 著) 清华大学出版社
- 高等物理化学 (刘寿长 石秋芝 著) 河南科学技术出版社 课后答案
- 化学工艺学_第二版_(米镇涛 著)_课后答案.化学工业出版社
- 小学生生理卫生 (杨培禾 著) 科学出版社 课后答案
- 传感器原理及应用_第三版_(王化祥_张淑英_)_天津大学_课后习题答案
- 谢希仁_计算机网络第五版习题答案
- ARM嵌入式系统基础教程 (周立功 著) 北京航天航空大学出版社 课后答案
- 传质分离过程 (刘家祺 著) 高等教育出版社 部分答案 课后答案
- 高分子化学第五版课后习题答案
相关文档
- 施工规范CECS140-2002给水排水工程埋地管芯缠丝预应力混凝土管和预应力钢筒混凝土管管道结构设计规程
- 施工规范CECS141-2002给水排水工程埋地钢管管道结构设计规程
- 施工规范CECS142-2002给水排水工程埋地铸铁管管道结构设计规程
- 施工规范CECS143-2002给水排水工程埋地预制混凝土圆形管管道结构设计规程
- 施工规范CECS145-2002给水排水工程埋地矩形管管道结构设计规程
- 施工规范CECS190-2005给水排水工程埋地玻璃纤维增强塑料夹砂管管道结构设计规程
- cecs 140:2002 给水排水工程埋地管芯缠丝预应力混凝土管和预应力钢筒混凝土管管道结构设计规程(含条文说明)
- cecs 141:2002 给水排水工程埋地钢管管道结构设计规程 条文说明
- cecs 140:2002 给水排水工程埋地管芯缠丝预应力混凝土管和预应力钢筒混凝土管管道结构设计规程 条文说明
- cecs 142:2002 给水排水工程埋地铸铁管管道结构设计规程 条文说明