- 2.21 MB
- 2022-04-22 11:24:41 发布
- 1、本文档共5页,可阅读全部内容。
- 2、本文档内容版权归属内容提供方,所产生的收益全部归内容提供方所有。如果您对本文有版权争议,可选择认领,认领后既往收益都归您。
- 3、本文档由用户上传,本站不保证质量和数量令人满意,可能有诸多瑕疵,付费之前,请仔细先通过免费阅读内容等途径辨别内容交易风险。如存在严重挂羊头卖狗肉之情形,可联系本站下载客服投诉处理。
- 文档侵权举报电话:19940600175。
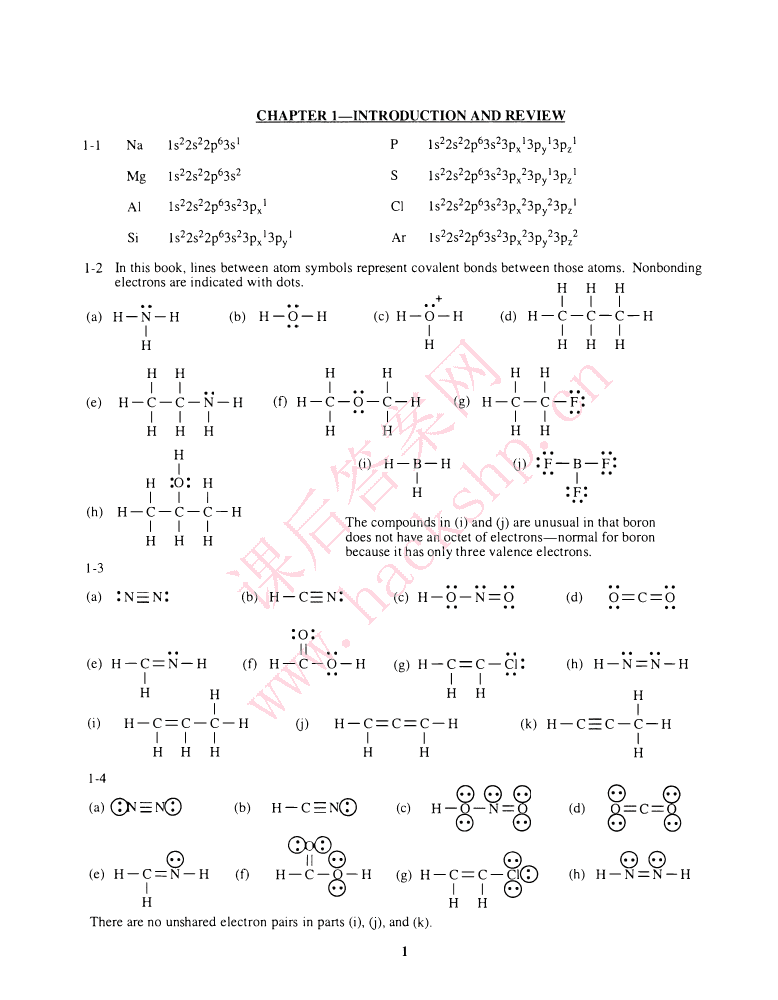
'CHAPTERI-INTRODUCTIONANDREVIEW1-1Na1s22s22p63s1P1s22s22p63s23px13py13pz1Mg1s22s22p63s2S1s22s22p63s23px23py13pz1AIIs22s22p63s23px1CI1s22s22p63s23px23py23pz1Si1s22s22p63s23px13pyIAr1s22s22p63s23px23py23pz21-2Inthisbook,linesbetweenatomsymbolsrepresentcovalentbondsbetweenthoseatoms.Nonbondingelectronsareindicatedwithdots.+HHHIII(a)H-N-H(b)H-O-H(c)H-O-H••(d)H-C-C-C-HIIIIIHHHHHHHHHHHIII••III(e)H-C-C-N-H(f)H-C-O-C-H(g)H-C-C-F:IIII••IIIHHHHHHHH(i)H-B-H(j):F-B-F:IH:0:HI--IH:F:(h)III..H-C-C-C-HThecompoundsin(i)and(j)areunusualinthatboronIIIHHHdoesnothaveanoctetofelectrons-normalforboronbecauseithasonlythreevalenceelectrons.1-3(a):NN:(b)课后答案网H-CN:(c)H-O-N=O(d)O==C=O:0:II(e)H-C=N-H(f)H-C-O-H(g)H-C==C-CI:(h)H-N=N-HIII••HHHHHIwww.hackshp.cnI(i)H-C==C-C-H(j)H-C==C==C-H(k)H-CC-C-HIIIIIIHHHHHH1-488(.:)0)0)(a)G}J-NQ)(b)H-CNeD(c)H-O-N=O(d)o==c=o0)0)0)0)C)J(D•8II·8888(e)H-C=N-H(f)H-C-O-H(g)H-C==C-CleD(h)H-N=N-HI8II8HHHTherearenounsharedelectronpairsinparts(i),(j),and(k).1
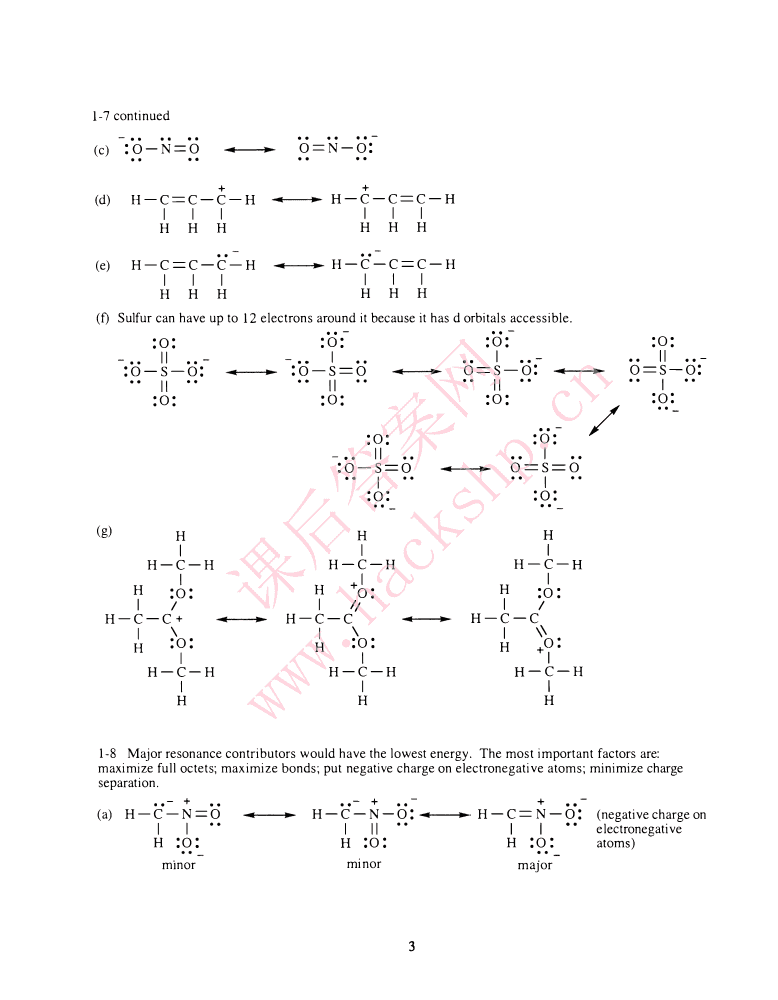
1-5Thesymbols"8+"and"8-"indicatebondpolaritybyshowingpartialcharge.(Inthearrowsymbolism,thearrowshouldpointtothepartialnegativecharge.)������(a)C-C1(b)C-O(c)C-N8+8-8-8+8-8+(g)N-O(h)N-S(i)N-B1-6Non-zeroformalchargesareshownbesidetheatoms.HHHHH11+..-11+1..-(a)H-C-O-H(b)H-N-H:C1:(c)H-C-N-C-H:Cl:11+1111HHHHHHIn(b)and(c),thechlorineispresentaschlorideion.Thereisnocovalentbondbetweenchlorineandotheratomsintheformula.HH-••I+(g)Na+H-B-H1-(d)Na+:O-C-H(e)H-C-H(f)H-C-HI11IHHHHHH1-H,/H1+(h)Na+H-B-C-N:(i)H-C-O/-C-HU)H-O-N-HI1+"IHH••_••HH:F-B-1F:HH,1/H课后答案网:..F:Asshownin(d),(g),(h),and(k),CalkalimetalslikesodiumandHpotassiumformonlyionicbonds,-·••O-C-C1/-HH-C=O-Hnevercovalentbonds.•(I)+••I"H1CHH/1"HHwww.hackshp.cn1-7Resonanceformsinwhichallatomshavefulloctetsarethemostsignificantcontributors.Inresonanceforms,ALLATOMSKEEPTHEIRPOSITIONS-ONLYELECTRONSARESHOWNINDIFFERENTPOSITIONS...-..-:0::0::0:"-..11••-(a):O-C-O:....:O-C=O..O=C-O:..-:0::0::0:"..--..1I••-(b):O-N-O:....:O-N=O...O=N-O:+++2
1-7continued(c):O-N=O..-....O=N-O:++(d)H-C=C-C-H....H-C-C=C-HIIIIIIHHHHHH(e)H-C=C-C-H....H-C-C=C-HIIIIIIHHHHHH(f)Sulfurcanhaveupto12electronsarounditbecauseithasdorbitalsaccessible.:0::..0:-:..0:-:0:IIIIII••-:O-S-O:....:O-S=O....O==S-O:....O=S-O:IIIIIII:0::0::0::0:..:0::..0:-/-III:O••-S=O....O==S==OII:0::0:(g)HHHIIIH-C-HH-C-HH-C-HH:0I:H+IH:0:I0:II课后答案网I1/IIH-C-C+....H-C-C....H-C-CIII\H:0:H:0:H+0:IIIH-C-HH-C-HH-C-HIIIHwww.hackshp.cnHH1-8Majorresonancecontributorswouldhavethelowestenergy.Themostimportantfactorsare:maximizefulloctets;maximizebonds;putnegativechargeonelectronegativeatoms;minimizechargeseparation.•-+..-++(a)H-C-N•=O....H-C-N-O:....H-C=N-O:(negativechargeonIIIIIIIelectronegativeH:0:H:0:H:0:atoms)mInorminormajor3
1-8continuedHHH++++(b)C=C-N=O•..C=C-N-O:...C-C=N-O:/11/111/11HH:0:HH:0:HH:0:majormajorminorThesetwoformshaveequivalentenergyandaremajorbecausetheyhavefulloctets,morebonds,andlesschargeseparationthantheminorcontributor.+(c)H-C=O-H..•H-C-O-H+11HHmajormmor(octets,morebonds)++(d)H-C=N=N:...-H-C-NN:11HHmajor(negativechargeminoronelectronegativeatom)(e)H-C-CN:..•H-C=C=N:11HHminormajor(negativechargeonelectronegativeatom)++(f)H-N-C-C=C-N-H"•H-N=C-C=C-N-H1111111111HHHHH课后答案网HHHHHmmormajorthesetwoformsaremajorcontributorstbecauseallatomshavefulloctets+••+H-N-C=C-C-N-H..•H-N-C=C-C=N-H11111www.hackshp.cn11111HHHHHHHHHHmmormajor..-:0::0::0::0::0::0:II••II1IIII1(g)H-C-C-C-H-----H-C=C-C-H-----H-C-C=C-H-111HHHmITIormajormajorthesetwohaveequivalentenergyandaremajorbecausethenegativechargeisonthemoreelectronegativeoxygenatom4
1-8continued..:0::0:-III+N(h)H-C-N-H......II----I..�H-C=-HIImajorHminorH(nochargeseparation)1-9YourLewisstructuresmayappeardifferentfromthese.Aslongastheatomsareconnectedinthesameorderandbythesametypeofbond,theyareequivalentstructures.Fornow,theexactplacementoftheatomsonthepageisnotsignificant.ALewisstructureis"complete"withunsharedelectronpairsshown.HHHHHHHHHIIIIIIIII(a)H-C-C-C-C-C-C-H(b)H-C-C-C-Cl:IIIIIIIIIHHHHCHHCH//H1"HH1"HHHHH:0:HHH:0:IIII/IIII(c)H-C-C-C-C=C(d)H-C-C-C-HIIIIIHHHHHHAlwaysbealertfortheimplieddoubleortriplebond.RememberthatChastohavefourbonds,nitrogenhasthreebonds,oxygenhastwobonds,andhydrogenhasonebond.Theonlyexceptionstothesevalencerulesarestructureswithformalcharges.H:0:HHHIII,1/(e)H-C-C-CN:C:0:课后答案网HI,IIIH(f)H-C-C-C-O-H/HICHH:0:HH/H1"HIIIIIIH(g)H-C-C-C-C-C-HIIIIwww.hackshp.cnHHHH1-10CompleteLewisstructuresshowallatoms,bonds,andunsharedelectronpairs.(a)HH(b)HHH(c)HHHHI/,I1/HIIH,C/C-HH-CC-HC-C,/,/,/H-CC-HCII\HH,C,CH-CI�IC-H/../••�C-HH_I1/HN"H/"N",-C-CI"HIHIHHIHHHH......CC......H/,••0,.......,H5
1-10continued·0·()eHH··(f)HHH,,/IIH,,/••"/C,/C,"/C....",...-0H-CCHH-CC/··IIIIIH-C,........C,H-C,/.C,H/CH/C/"H/"-HHHIH(g)H:0:H(h)H:0:HHHIIIIIIIIIIH/C:--..,/,C-C-HH-C-C-C-C-C-H"c"cIIIIIIIIHHHHHH/C,C"iC,HIH1-11Thereisoftenmorethanonecorrectwaytowritecondensedstructuralformulas.Youmustoftenmakeinferencesaboutwhatacondensedformulameansaccordingtovalencerules,especiallyinstructureswithC=Oasshowninparts(a)and(d).(a)CH3COCH2CH2CH3(b)(CH3hCHCH2CH20H(c)(CH3hCHCH2CH(OH)CH3(the0hasadoublebondtothecarbonprecedingit)orCH3CH(CH3)CH2CH20HorCH3CH(CH3)CH2CH(OH)CH3(d)CH3CH2CH(CH3)CH2CHO(the0hasadoublebondtothecarbonprecedingit)1-12Ifthepercentvaluesdonot课后答案网sumto100%,theremaindermustbeoxygen.Assume100gofsample;percentsthentranslatedirectlytogramsofeachelement.Thereareusuallymanypossiblestructuresforamolecularformula.Yoursmaybedifferentfromtheexamplesshownhere.(a)40.0gCsomepossiblestructures:=3.33molesC3.33moles=1C12.0g/molewww.hackshp.cnH°HIIII6.67gHHO-C-C-C-OH1.01g/mole=6.60molesH3.33moles=1.98==2HII53.33gOHH16.0g/mole=3.33moles°3.33moles=1°OHempiricalformula=�c::::::>empiricalweight=30molecularweight=90,threetimestheempiricalweightc:::::>HoAOHthreetimestheempiricalformula=molecularformula=IC3H603IMANYotherstructurespossible.6
1-12continued(b)32.0gCsomepossiblestructures:12.0g/mole=2.67molesC1.34moles=1.99::=2CHHII6.67gHH-C-C-N026.60molesH-:-1.34moles=4.935Hl.01g/moleIIIS.7HHgN14.0g/mole=1.34molesN-:-1.34moles=1NHaH42.6IIIga_.16.0g/mole-2.66molesa-;-1.34moles=1.99::=2aN-C-O-C-H/IHHempiricalformula=IC2HsN02Ic:::::::>empiricalweight=75MANYothermolecularweight=75,sameastheempiricalweightc:::::::>structurespossible.empiricalformula=molecularformula=C2HsN02I(c)37.2gC12.0g/mole=3.10molesC-:-1.55moles=2C7.75gH1.01g/mole=7.67molesH-:-1.55moles=4.95::=5HThereisonlyonestructurepossiblewiththismolecular3555.0.45gg/moleCI_-1.55molesCl-;-.1.55moles-1_CIformula:HHempiricalformula=IC2HsClIc:::::::>empiricalweight=64.46IIH-C-C-ClIImolecularweight=64,sameastheempiricalweightc:::::::>HH课后答案网empiricalformula=molecularformula=IC2HsCII(d)3S.4gC12.0g/mole=3.20molesC1.60moles2Csomepossiblestructures:www.hackshp.cnHH·II1.���/�:le=4.75molesH-:-1.60moles=2.973HH-C-C==C-C-HIIIIHClClH3��4���ole=1.60molesCI-:-1.60moles=1ClClC]empiricalformula=IC2H3ClIc:::::::>empiricalweight=62.45Umolecularweight=125,twicetheempiricalweightc:::::::>Cl�twicetheempiricalformula=molecularformula=""-C-4-H6-C-12---o.,MANYotherCl7structurespossible.
1-131moleHEr(a)5.00gHBrx=0.0618molesHBr80.9gHBr0.0618molesHEr0.0618molesH30+(100%dissociated)0.0618molesH30+1000mL0.618molesH30+100x1L=1LsolutionmLpH=-10gIO[H30+]=-loglo(0.618)=�1moleNaOH(b)1.50gNaOHx=0.0375molesNaOH40.0gNaOH0.0375molesNaOH0.0375moles-OH(100%dissociated)0.0375moles-OH1000mL0.75moles-OH50.xlL=1Lsolution=0.75MmL[H30+]=1x10-14=1x10-14=1.33x10-14[-OH]0.75(thenumberofdecimalplacespH=-loglO[H30+]=-loglo(1.33x10-14)inapHvalueisthenumberofsignificantfigures)1-14(a)Bydefinition,anacidisany课后答案网speciesthatcandonateaproton.Ammoniahasaprotonbondedtonitrogen,soammoniacanbeanacid(althoughaveryweakone).Abaseisaprotonacceptor,thatis,itmusthaveapairofelectronstosharewithaproton;intheory,anyatomwithanunsharedelectronpaircanbeabase.Thenitrogeninammoniahasanunsharedelectronpairsoammoniaisbasic.Inwater,ammoniaistooweakanacidtogiveupitsproton;instead,itactsasabaseandpullsaprotonfromwatertoasmallextent.(b)waterasanacid:H2O--+www.hackshp.cnNH3-OH+NH4+waterasabase:H2O+HClH3O++Cl-(c)methanolasanacid:CH30H+NH3CH3O-+NH4+methanolasabase:CH30H+H2SO4CH3OH2++HS04-8
1-15.-HCOO-(a)HCOOH+-CN--+HCNFAVORSstrongerstrongerweakerweakerPRODUCTSacidbasebaseacidpKa3.76pKa9.22(b)--CH3COOHCH3COO-+CH30H..+CH3O-FAVORSweakerweakerstrongerstrongerREACTANTSbaseacidacidbasepKa15.5pKa4.74(c)CH30H.-CH30-Na+NH3FAVORS+NaNH2--+strongerstrongerweakerweakerPRODUCTSacidbasebaseacidpKa15.5pKa33Na+-OCH3.-FAVORS(d)+HCN--HOCH3+NaCNstrongerstrongerweakerweakerPRODUCTSbaseacidacidbasepKa9.22pKa15.5(e)HCl.-H3O+CI-+H2O--+FAVORSstrongerstrongerweakerweakerPRODUCTSacidbaseacidbaseThefirstreactioninTable1-5showstheKeforthisreactionis160,favoringproducts.q.-(f)H30++CH3O---H2O+CH30HFAVORSstrongerstronger课后答案网weakerweakerPRODUCTSacidbasebaseacidpKa-1.7pKa15.5TheseventhreactioninTable1-5showstheKeforthereverseofthisreactionis3.2x10-16.qTherefore,Keforthisreactionaswrittenmustbetheinverse,or3.1x1015,stronglyfavoringqproducts.www.hackshp.cn1-16:0::0:IIIICH3-C-O-HCH3-C-O-H••+HProtonationofthedouble-bondedoxygengivesthreeresonanceforms(asshowninSolvedProblem1-5(c));protonationofthesingle-bondedoxygengivesonlyone.Ingeneral,themoreresonanceformsaspecieshas,themorestableitis,sotheprotonwouldbondtotheoxygenthatgivesamorestablespecies,thatis,thedouble-bondedoxygen.9
1-l7InSolvedProblem1-4,thestructuresofethanolandmethylamineareshowntobesimilartomethanolandammonia,respectively.Wemustinferthattheiracid-basepropertiesarealsosimilar.(a)Thisproblemcanbeviewedintwoways.1)Quantitatively,thepKavaluesdeterminetheorderofacidity.2)Qualitatively,thestabilitiesoftheconjugatebasesdeterminetheorderofacidity(seeSolvedProblem1-4forstructures):theconjugatebaseofaceticacid,acetateion,isresonance-stabilized,soaceticacidisthemostacidic;theconjugatebaseofethanolhasanegativechargeonaveryelectronegativeoxygenatom;theconjugatebaseofmethylaminehasanegativechargeonamildlyelectronegativenitrogenatomandisthereforetheleaststabilized,somethylamineistheleastacidic.aceticacid>ethanol>methylaminepKa4.74pKa""15.5pKa33strongestacidweakest""acid(b)Ethoxideionistheconjugatebaseofethanol,soitmustbeastrongerbasethanethanol;SolvedProblem1-4indicatesethoxideisanalogoustohydroxideinbasestrength.MethylaminehaspKb3.36.Thebasicityofmethylamineisbetweenthebasicityofethoxideionandethanol.ethoxideion>methylamine>ethanolstrongestbaseweakestbase1-18Curvedarrowsshowelectronmovement,asdescribedintextsection1-14...CH3CH2-�:..__+CH3-�-HstrongeracidstrongerbaseconjugatebaseHweakerbaseconjugateacidequilibriumfavorsPRODUCTSweakeracid"0"H"11".��1(b)CH3CH2-C-0-H••++CH3-N-CH3CH3-�-CH3--strongeracid••课后答案网HIHstrongerbaseconjugateacidweakeracidequilibriumfavorsPRODUCTSwww.hackshp.cnconjugatebase•O"Hweakerbase(c)CH3-O..-H�0."11"••..CH3-O1+-H+H-O-S-O-H--+stronger••base:0II·:conjugate••acidstrongeracidweakeracidequilibriumfavorsi-··:�:..:0:PRODUCTSII..,..:O-S-O-H........l----I..�O==S-O-H..O==S-O-H••II••I••II••:0::0::0:..:0:-rconjugatebase,weakerbase10
1-18continued(d)-..�0·..Na+:O-H+H-S-H..---..H-O-H+Na+:S-Hstrongerbasestrongeracidconjugateacidconjugatebaseequilibrium_weakeracidweakerbasefavorsPRODUCTS__(e)HI�"+/"••-�-HCH3-��H+CH3-O:---CH3-+CH3-O-H••HstrongerbaseHconjugateacidstrongeracidconjugatebaseweakeracidequilibriumfavorsPRODUCTSweakerbase:0:II-••+CHi3-C-0..::••0:-tstrongeracidstrongerbaseconjugateacid�Iweakeracid..........CH3-C=O••equilibriumfavorsPRODUCTSconjugatebase••weakerbase(g):0::0::0::0:-}II.�-..IIIIICH-C-O-H••3+:O-S-CH3.......f---l...�0==S-CH3.......f---..._0==S-CH3..••II••I••IIweakeracid~:0::0::0:weakerbaseequilibriumfavorsREACTANTS课后答案网:0::0::0:IIIIICH3-C-�:......f---l...�CH3-C=�H-O-S-CH3••IIconjugatebase:0:strongerbaseconjugateacidwww.hackshp.cnstrongeracid1-19Solutionsfor(a)and(b)arepresentedintheSolvedProbleminthetext.Here,thenewlyformedbondsareshowninbold.HI_(c)H-B-H+CH3-O/"••-CH3"H-B-H1"-1+acidH�baseCH3-O-CH3••..(d)co::0:IIICH3-C�-H+:O-H••CH3-r-H••acidbase:O-H11
1-19continued(e)Bronsted-Lowry--c-protontransfer�:�::�:H:0:..H-C-C-H+/..:O-H�==�H-C-C..-H....-...H-b==b-H+H-O-Hi-�••_}k.)�baseacid..�()..-(f)CH3-�-H+CH3-S=!:+:Cl:Hacidbase1-20Learningorganicchemistryissimilartolearningaforeignlanguage:newvocabulary,newgrammar(thereactions),somenewconcepts,andevenanewalphabet(thesymbolismofchemistry).Thistypeofdefinitionquestionisintendedtohelpyoureviewthevocabularyandconceptsineachchapter.AllofthedefinitionsandexamplesarepresentedintheGlossaryandinthechapter,sothisSolutionsManualwillnotrepeatthem.Usethesequestionstoevaluateyourcomprehensionandtoguideyourreviewoftheimportantconceptsinthechapter.1-21(a)CARBON!(b)oxygen(c)phosphorus(d)chlorine1-22valencee----12345678H课后答案网He(2e-)LiBeBCN0FNepSClBrwww.hackshp.cnI1-23(a)ioniconly(b)covalent(H-O-)andionic(Na+-OH)(c)covalent(H-CandC-Li),buttheC-Libondisstronglypolarized(d)covalentonly(e)covalent(H-CandC-O-)andionic(Na+-OCH3)(f)covalent(H-CandC=OandC-O-)andionic(HC02-Na+)(g)covalentonly12
1-24:ClCl::ClCl::ClCl::ClCl:..,../...."/....,../....,/..(a)P••p............(b)N••N..........:CIl::Cl/":CIl:Cl::CIl::..c!"":CII:Cl:.•CANNOTEXISTNClsviolatestheoctetrule;nitrogencanhavenomorethaneightelectrons(orfouratoms)aroundit.Phosphorus,athird-rowelement,canhavemorethaneightelectronsbecausephosphoruscanusedorbitalsinbonding,soPClsisastable,isolablecompound.1-25YourLewisstructuresmaylookdifferentfromthese.Aslongastheatomsareconnectedinthesameorderandbythesametypeofbond,theyareequivalentstructures.Fornow,theexactplacementoftheatomsonthepageisnotsignificant.HHH,1/CHH.(a)H-N-N-H(b)H-N=N-H(c)H-,C-N-C-H1+/:CI.:-/"IIHIHHHC/1"HHHHH:0:H:0:HIIIIIIII(d)H-C-CN:(e)H-C-C-H(f)H-C-S-C-HIIIIHHHH:0:HH:0:HIIIIIII(g)H-O-S-O-H(h)H-C-N=C=O(i)H-C-O-S-O-C-HII课后答案网IIIII:0:HH:0:HHHHH/,,1/H:NHCHIIII"IH-C-C-C-H(k)H-C-C-N=OIIwww.hackshp.cnH/IHHC/1"HHH1-26HH:0:H:0:H:0:IIII.•IIIIII(a)H-C-C=C-C-C=C-C-O-H(b):NC-C-C-C-C-HIIIIIIIIHHHHHHHH13
1-26continuedH-O..:H:0:IIII(c)H-C=C-C-C-C-O-HIIII••HHHH1-27Ineachsetbelow,thesecondstructureisamorecorrectlineformula.Sincechemistsarehuman(surprise!),theywilltakeshortcutswherepossible;thefirststructureineachpairusesacommonabbreviation,eitherCOOHorCHO.MakesureyouunderstandthatCOOHdoesnotstandforC-O-O-H.LikewiseforCHO.�/"...�(a)/"��.....COOH(b)NC�CHOoOHHORORN-CTIYoo0(c)�COOHOHHOH课后答案网CHOOROR�OH01-28HHHHwww.hackshp.cnHHH1I1111Ithesearetheonlytwopossibilities,(a)H-C-C-C-C-HandH-C-C-C-HbutyourstructuresmayappearII1II11different-makingmodelswillHHHHHCHhelpyouvisualizethesestructuresH/1"HHHHHHHHII1IIIthesearetheonlytwopossibilities,(b)H-C-C-N:andH-C-N-C-HbutyourstructuresmayappearIIIIIdifferent-makingmodelswillHHHHHhelpyouvisualizethesestructures14
1-28continued(c)Thereareseveralotherpossibilitiesaswell.Youranswermaybecorrectevenifitdoesnotappearhere.Checkwithothersinyourstudygroup.HHHHHHHHHIIIIIIIII:O-C-C-C-O::O-C-C-O-C-H:O-C-C-C-HIIIIIIIIIIIIIHHHHHHHHHHH:0:HIHH:0::0:III/Thesearetheonlythree(d)H-C-C-HH-C=C-O-HH-C-C-HstructureswiththismolecularIIIIIformula.HHHHH1-29HHHHHHHHHIIIIIIIII(a)onlythreeO-C-C-C-HH-C-C-O-C-HH-C-C-C-HpossibleIIIIIIIIIIstructuresHHHHHHHH0HIHOCH2CH2CH3CH3CH20CH3HCH3CH(OH)CH3(b)Thereareseveralotherpossibilitiesaswell.HH0H0HHHIIIIIIIIIIH-C-C-C-HH-C-C-C-HH-C=C-C-O-HH-C=C-C-HIIIIIIIIIIHHHHHHHH0HICH3CH2CHO课后答案网CH3COCH3H2C=CHCH20HHHH2C=C(OH)CH3IH-C=C-O-C-HIIIHHHH2C=CHOCH3www.hackshp.cn1-30Generalrule:molecularformulasofstablehydrocarbonsmusthaveanevennumberofhydrogens.TheformulaCH2doesnothaveenoughatomstobondwiththefourorbitalsofcarbon.onecarbon:HHHIItwocarbons:H-CC-HH-C=C-HH-C-C-HIH-C-HIIIIC2H4HHC2H6HHIHCH4HHHHHIIIIIthreecarbons:H-CC-C-HH-C=C-C-HH-C-C-C-HIIIIIIIC3H4HC3H6HHHC3HSHHH15
HH(b)H.....�_�_HHIH.....CC..../,00/"HNHIHHHI/C,,....HHHH:0::NC,III"HIIHIH�H-N-C-C-C-C-O-H(e)/C,IHIIIIH-CC-HHHHHHHIII,....HH-C-CCHI1"00//HHO-C-C-H(g)HH"IC_HHC-C:0:H,II\"IH-C-CC-S==OHHH/C=C/I(h):0:·00,,·0:0:IIHHIHIH,/C,I/C,ooHCCO-H\IC-C-H/HIH1-32(a)CsHsN(b)C4H9N(c)C4H9NO(f)C9H1SO(g)C7Hs03S(h)C6H6031-33课后答案网(c)somepossiblestructures-MANYotherstructurespossible:(a)100%-62.0%C-10.4%H=27.6%oxygenHH62.0gC_5.l7molesC-;-.l.73moles_-2.99_-3CH,""C,"H12.0g/mole-H-CC-O-HII10.4gHH-"C"c"C-O-H,l.01g/mole=10.3molesHwww.hackshp.cn-;-l.73moles=5.95==6HH""HHHle=l.73moles°-;-l.73moles=1°HHHHH°1��06g7�oIIIIIIIH-C-C-C-C-C-C-O-H(b)empiricalformulaIIIII=IC3H60Ic:::::>empiricalweight=58HHHHHHHHHH0molecularweight=117,aboutdoubletheempiricalweightI1IIIIIH-O-C-C-C-C-C-C-HqdoubletheempiricalformulaIIII1=molecularformula=HHHHHC6H1202IHHHH0HIIIIIIIH-C-C-C-C-C-O-C-HIIIIIHHHHH16
1-34Non-zeroformalchargesareshownbytheatoms.HHH+••-+,1/(a)H-C=N=N:......I----I..�H-C-N-N:(b)CH11,+1••HHH-C-N-O:HHHH/I+1+I1C(c)H-C=C-C-H(d)H-C-N=O(e)H-C-O-C-HH/1"H11111I+11HHHHH:0:HCH..-/1"HHH1-35Thesymbols"8+"and"8-"indicatebondpolaritybyshowingpartialcharge.Electronegativitydifferencesgreaterthanorequalto0.5areconsideredlarge.8+8-8-8+8-8+8+8-8+8-(a)C-CI(b)C-H(c)C-Li(d)C-N(e)C-Olargesmalllargesmalllarge8-8+8-8+8-8+8-8+8+8-(f)C-B(g)C-Mg(h)N-H(i)O-H(j)C-Brlargelargelargelargesmall1-36Resonanceformsmusthaveatomsinidenticalpositions.Ifanyatommovesposition,itisadifferentstructure.(a)differentcompounds-ahydrogenatomhaschangedposition(b)resonanceforms-onlythepositionofelectronsisdifferent(c)resonanceforms-onlythepositionofelectronsisdifferent(d)resonanceforms-onlythepositionofelectronsisdifferent(e)differentcompounds-ahydrogenatomhaschangedposition(f)resonanceforms-onlythe课后答案网positionofelectronsisdifferent(g)resonanceforms-onlythepositionofelectronsisdifferent(h)differentcompounds-ahydrogenatomhaschangedposition(i)resonanceforms-onlythepositionofelectronsisdifferent(j)resonanceforms-onlythepositionofelectronsisdifferent1-37(a)Hwww.hackshp.cn..:0:H:0:IIIiIH-C-C-C-H....H-C-C=C-HI1I1HHHH(b):0::0::0:IIIIIH-C-C=C-C-H.......I----J..�H-C-C-C=C-H.......f----J..�H-C=C-C=C-HIIII1I11IHHHHHHHHH17
1-37continued+(c)4<>CH2•OCH,-+()CH,0-�/;CH2...0/�CH2++(d)0+...0----0+��..(e)<>�:•40.0.-----:0=..0-/-<>�:-o�++-H(f)+(>-H4•CN-H-CN+•(g)0课后答案网+00....o----0�0+....(h)_.�_QQwww.hackshp.cnQ:0::0::0:..-+CH3-C=C-C=C-C-C+H3CH3-C=C-C-C=C-CH(i)-----3IIIII/IIIIIHHHHHHHHHH+CH3-C-C=C-C=C-CH3IIIIIHHHHH(j)noresonancefonns-thechargemustbeonanatomnexttoadoubleortriplebond,ornexttoanonbondedpairofelectrons,inorderforresonancetodelocalizethecharge18
1-38......(a)O==S-O:..•:O-S==O..•O==S==O++(b)0=0-0:..•:0-0=0++(c)ThelastresonanceformofS02hasnoequivalentformin03.Sulfur,athirdrowelement,canhavemorethaneightelectronsarounditbecauseofdorbitals,whereasoxygen,asecondrowelement,mustadherestrictlytotheoctetrule.1-39#3�..(a)#1NH#2�••II••/CH3-N-C-NH2IH�H+to#2,IH+to#3�HNHNH+II+IIINH2+CH3-N-C-NH2IICH3-N-C-NH3ICH3-�-C-NH2IHHnootherresonanceformsHnootherresonanceformstNH2NH2NH2+III+CH3-N=C-NH2.......t---"l...CH3-N-C-NH2.......t---"l....CH3-N-C=NH2II+IHHH(b)Protonationatnitrogen#3课后答案网givesfourresonanceformsthatdelocalizethepositivechargeoverallthreenitrogensandacarbon-averystablecondition.Nitrogen#3willbeprotonatedpreferentially,whichweinterpretasbeingmorebasic.1-40(a)CH3-C-CN:......t----Iwww.hackshp.cn....CH3-C==C=N:II••HHminormajor(negativechargeonelectronegativeatom)..-:0::0::0:I+III(b)CH3-C==C-C-CH3......t----I...CH3-C-C==C-CH3......t----I...CH3-C-C==C-CH3II+IIIIHHHHHHminormmormajor-fulloctets,nochargeseparation19
1-40continued..-..-(c):0::0::0::0::0::0:II-..IIIIIIIICH3-C-?-C-CH3........1-----1...CH3-C==C-C-CH3.......1-----1...CH3-C-C==C-CH3IIHHHminormajormajor}�-----------------�--_/Ynegativechargeonelectronegativeatoms-equalenergy(d)+-++••CH3-C-C==C-N==0-CH3-C==C-C-N=0-CH3-C==C-C==N-0:IIII••IIII••IIII••HHH:0:HHH:0:HHH:0:minorminormajor-negativechargeonelectronegativeatomsNOTE:Thetwostructuresbelowareresonanceforms,varyingfromthefirsttwostructuresinpart(d)bythedifferentpositionsofthedoublebondsintheN02.Usually,chemistsomitdrawingthesecondformoftheN02groupalthoughweallunderstandthatitspresenceisimplied.Itisgoodideatodrawalltheresonanceformsuntiltheybecomesecondnature.Theimportanceo/understandingresonanceformscannotbeoveremphasized.+-+CH3-C-C==C-N-0:.......f---.�CH3-C==C-C-N-0:••IIIII••IIIII••HHH:0:HHH:0:+NH2NH2NH2IIII+(e)CH3CH2-C-NH2......I--课后答案网-J.,..CH3CH2-C-NH2.......I---J.�CH3CH2-C==NH2+mmor..major-fulloctetsmajor-fulloctet:;y1-41equalenergy(a)+++CH3-C-CH3CH3-C-0-CH3www.hackshp.cn......I---l.,�CH3-C==0-CH3II••I••HHHnoresonancestabilizationmorestable-resonancestabilizedH�)++I+CH2==C-C-CH3.......f---l.,�CH2-C==C-CH3CH2==C-C-CH2IIIIIIHHHHHHmorestable-resonancestabilizednoresonancestabilization20
1-41continued(c)H-C-CH3H-C-C-N:.....H-C=C=N:\HHHnoresonancestabilizationmorestable-resonancestabilized+./CH2c"HC...:/CC"Hmorestable-resonancestabilizedHnoresonancestabilization(e)H+CH3-N-CH3CH3-�-CH3CH3-C-CH31........I----I..�ICH3-C-CH3CH3-C-CH3CH3-C-CH3++morestable-resonancestabilizednoresonancestabilization1-42ThesepKavaluesfromthetext,Table1-5,andAppendix5providetheanswers.ThelowerthepKa"thestrongertheacid.leastacidicmostacidicNH3<<<331-43Conjugatebasesoftheweakestacidswillbethestrongestbases.ThepKavaluesoftheconjugateacidsarelistedhere.(TherelativeorderofthefirsttwowasdeterminedfromthepKavaluesofsulfuricacidandprotonatedacedicacidinAppendix课后答案网5ofthetextbook.)leastbasicmostbasicfrom�6.1from�5from4.74from15.5from15.7from331-44(a)pKa=�loglOKa=�logwww.hackshp.cnlO(5.2x10-5)=4.3forphenylaceticacidforpropionicacid,pKa4.87:Ka=10-4.87=1.35x10-5(b)phenylaceticacidis3.9timesstrongerthanpropionicacid5.2x10-5=3.91.35xl0-S(c)<>CH2COO-+CH3CH2COOH...--<>CH2COOH+CH3CH2COOweakeracidstrongeracidEquilibriumfavorstheweakeracidandbase.Inthisreaction,reactantsarefavored.21
1-45Thenewlyformedbondisshowninbold...-("....-(a)CH3-O..:�+CH3-CI..:..CH3-�-CH3+:CI:nucleophileelectrophileLewisbaseLewisacid(b)CH3-O-CH3+H-O-HCH3-?-CH3+H+�-H+1)••H3c�ucleoPhileCH3eIectrophOIeILewisbaseLewisacid(c)..-c�::0:�1H-C-H+:N-HH-C-H"----/11+HH-N-Helectrophilenucleophile1LewisacidLewisbaseHHCH3+-N-1CH2CH3..-+:CI:nucleophileelectrophileH1LewisbaseLewisacid+·0·�:0::O-H:0:",,"�"""(e)CH3-C-CH3+H-�O-.S-OH..---t..�CH3-C-CH3+:O-S-OH....nucleophile课后答案网""Lewisbase:0::0:electrophileLewisacidCI1-www.hackshp.cn+:Cl-Al-Clnucleophileelectrophile1LewisbaseLewisacidClThismayalsobewrittenintwosteps:associationoftheCIwithAI,andasecondstepwheretheC-Clbondbreaks...-:R�-:0:..I(g)CH3-C-CH2+:O-H---t..�CH3-C==CH2+H-O-H"--I�J••H�nucleophileelectrophileLewisbaseLewisacid22
1-45continu�F-I+(h)F-B-FCH2=CH2•F-f-CH2-CH2IFnucleophileFelectrophileLewisbaseLewisacid+�+(i)BF3-CH2-CH2+CH2=CH2..BF3-CH2-CH2-CH2-CH2electrophilenucleophileLewisacidLewisbase1-46(a)H2S04+CH3COO-HS04-+CH3COOH+(b)CH3COOH+(CH3hN:CH3COO-+(CH3hN-Hoo(e)<)g-O-H+�OH<)g-o-+H20+(d)(CH3hN-H+-OH(CH3hN:+H20ooIIII(e)HO-C-OH+2-课后答案网0H-O-C-O-+2H20(f)H20+NH3HO-++NH4(g)HCOOH+CH30-HCOO-+CH30Hwww.hackshp.cn1-47(a)CH3CH2-O-H+CH3-Li-----l.�CH3CH2-0-Li++CH4(b)TheconjugateacidofCH3LiisCH4Table1-5givesthepKaofCH4as>40,oneoftheweakestacidsknown.Theconjugatebaseofoneoftheweakestacidsknownmustbeoneofthestrongestbasesknown.23
1-48FromtheamountsofCO2andH20generated,themilligramsofCandHintheoriginalsamplecanbedetermined,thusgivingbydifferencetheamountofoxygeninthe5.00mgsample.Fromthesevalues,theempiricalformulaandempiricalweightcanbecalculated.(a)howmuchcarbonin14.54mgCO21mmoleCO21mmoleC12.01mgC14.54mgCO2xxX=3.968mgC44.01mgCO21mmoleCO21mmoleChowmuchh)::drogenin3.97mgH2Q1mmoleH2O2mmolesH1.008mgH3.97mgH20xxX=0.444mgH18.016mgH2O1mmoleH2O1mmoleHhowmuchox)::genin5.00mgestradiol5.00mgestradiol-3.968mgC-0.444mgH=0.59mg°calculateempiricalformula3.968mgC=0.3304mmolesC0.037mmoles=8.93==9C12.01mg/mole0.444mgH=0.440mmolesH0.037mmoles=11.9""12H1.008mg/mole0.59mg°=0.037课后答案网mmoles°0.037mmoles=1°16.00mg/moleempiricalformula=�empiricalweight=136(b)molecularweight=272,exacwww.hackshp.cntlytwicetheempiricalweighttwicetheempiricalformula=molecularformula=24
CHAPTER2-STRUCTUREANDPROPERTIESOFORGANICMOLECULES2-1Thefundamentalprincipleoforganicchemistryisthatamolecule"schemicalandphysicalpropertiesdependonthemolecule"sstructure:thestructure-functionorstructure-reactivitycorrelation.Itisessentialthatyouunderstandthethree-dimensionalnatureoforganicmolecules,andthereisnobetterdevicetoassistyouthanamolecularmodelset.Youarestronglyencouragedtousemodelsregularlywhenreadingthetextandworkingtheproblems.(a)requiresuseofmodels(b)HH,fThewedgebondsrepresentbondscomingoutoftheplaneofthepaperHCHtowardyou.""""C/"C/"./Thedashedbondsrepresentbondsgoingbehindtheplaneofthepaper.JJ./HHHH2-2(a)Thehybridizationofoxygenissp3sinceithas(b)Theelectrostaticpotentialmapfortwosigmabondsandtwopairsofnonbondingelectrons.watershowsthatthehydrogenshaveThereasonthatthebondangleof104.5°islessthanthelowelectronpotential(blue),andthe3perfecttetrahedralangleof109.5°isthatthelonepairsinareaoftheunsharedelectronpairsinsp3thetwosporbitalsarerepellingeachothermoreorbitalshashighelectronpotential(red).stronglythantheelectronpairsinthesigmabonds,therebycompressingthebondangle.highelectron(0"potential(red)repulsion(0OOO,�)lowelectronO""Hpotential(blue).H)compression22-3Eachdouble-bondedatomissphybridizedwithbondanglesabout120°;geometryaroundsp2atoms2istrigonalplanar.In(a),allfourcarbonsandthetwohydrogensonthespcarbonsareallinoneplane.3Eachcarbonontheendissphyb课后答案网ridizedwithtetrahedralgeometryandbondanglesabout109°.In(b),thetwocarbons,thenitrogen,andthetwohydrogensonthesp2carbonareallinoneplane.TheCH3carbonis3sphybridizedwithtetrahedralgeometryandbondanglesabout109°.(b)www.hackshp.cn2-4Thehybridizationofthenitrogenandthetriple-bondedcarbonaresp,givinglineargeometry(C-C-Narelinear)andabondanglearoundthetriple-bondedcarbonof180°.TheCH3carbonis3sphybridized,tetrahedral,withbondanglesabout109°.25
2-5(a)linear,bondangle180°••••o==C==o••••+++Sp2SpSp2(b)allatomsaresp3;tetrahedralgeometryandbondanglesof109°aroundeachatomnotabond-showsnotabqnd-;-showsHHloneRaircoming•,".��lon�pairgomgI••Iout01paper""---/behmdpaperH-C-O-C-HH,.......0,.....H••CCII",HHlA""HHHH(c)allatomsaresp3;tetrahedralgeometryandbondanglesof109°aroundeachatomHHHH-C-N-C-H"../H�".....CHH/I"HI........--notabond-showsCHN"/.lonepairgoingbehindpaper/1""-...HHC/","".HJ"=:.,"C-HHHJHH(d)trigonalplanararoundthecarbon,bondangles120°;tetrahedralaroundthesingle-bondedoxygen,bondangle109°:0:,.....--·0·sp3IIallatomsinI..rCHII••/"••/oneplanesp2H-C-O-HH°••�..(e)carbonandnitrogenbothsp,课后答案网linear,bondangle180°H-CN:allthreeatomsinaline(0trigonalplanararoundthesp2carbons,bondangles120°;aroundthesp3carbon,tetrahedralgeometryand109°anglessp2Hwww.hackshp.cnH1H/IC==CH-C-C==C-H/IIIH/C"HHHH,"H3spH(g)trigonalplanar,bondangleabout120°(theotherresonanceformofozoneshowsthatBOTH..0==0-0....-·endoxygensmustbesp2_-••+••seeSolvedProblem2-8)26
2-6Carbon-2issphybridized.IftheporbitalsmakingthepibondbetweenC-landC-2areintheplaneofthepaper(puttingthehydrogensinfrontofandbehindthepaper),thentheotherporbitalonC-2mustbeperpendiculartotheplaneofthepaper,makingthepibondbetweenC-2andC-3perpendiculartothepaper.ThisnecessarilyplacesthehydrogensonC-3intheplaneofthepaper.(Modelswillsurelyhelp.)modelofperpendicularnbondsHH"""123/"C==C==CH"""tHsp2-7Forclarity,electronsinsigmabondsarenotshown.(a)carbonandoxygenarebothsp2hybridizedOnepairofelectronsonoxygenisalwaysinansp2orbital.Theotherpairofelectronsisshowninaporbitalinthefirstresonanceform,andinapibondinthesecondresonanceemptyform.orbital课后答案网(b)oxygenandbothcarbonsaresp2hybridizedH1200H\C==O........I---l..�C-O..:.1-..www.hackshp.cnII..H-·CH-C12�HH27
2-7continued(c)thenitrogenandthecarbonbondedtoitaresphybridized;theleftcarbonissp2H1800H-1200(c�..N:II��c-cN://HHoil(d)theboronandtheoxygensbondedtoitaresp2hybridizedH-O:HH-O:HH-OHH-O:HII+\IIB-O:II..B=O:II�B-O:..�B-O:••·•••II+I+IIH-O..:H-O:H-O:H-O......POSP"Q(POsP2eemptypeH-O(j""""""""0H{J"Q"""""""P""",JDH0课后答案网..",B-O_""""""B-�PO�o-06PH-O�sp"H-OVtQDsp"�Osp2Osp"www.hackshp.cn!PG/.:Sp2H-oo�""""",:n,:(.:)p""""�_VH���/O�-sp2H_+O••28
2-8Verycommonlyinorganicchemistry,wehavetodeterminewhethertwostructuresarethesameordifferent,andiftheyaredifferent,whatstructuralfeaturesaredifferent.Inorderfortwostructurestobethesame,allbondingconnectionshavetobeidentical,andinthecaseofdoublebonds,thegroupsmustbeonthesamesideofthedoublebondinbothstructures.(Agoodexercisetodowithyourstudygroupistodrawtwostructuresandaskiftheyarethesame;ordrawonestructureandaskhowtodrawadifferentcompound.)(a)differentcompounds;HandCH3ononecarbonofthedoublebond,andCH3andCH2CH3ontheothercarbon-sameinbothstructures;drawingaplanethroughtheporbitalsshowstheHandCH3areonthesamesideofthedoublebondinthefirststructure,andtheHandtheCH2CH3areonthesamesideinthesecondstructure,sotheyareDIFFERENTcompoundsHe3""CH3/-------e::E-C----------theseareDIFFERENTIH""CH2CH3............comparecompare(b)samecompound;inthestructureontheright,therightcarbonhasbeenrotated,butthebondingisidenticalbetweenthetwostructures(c)differentcompounds;HandBrononecarbon,FandCiontheothercarboninbothstructures;HandCIonthesamesideoftheplanethroughtheC=Cinthefirststructure,andHandFonthesamesideoftheplanethroughtheC=Cinthesecondstructure,sotheyareDIFFERENTcompounds(d)samecompound:inthestructureontheright,therightcarbonhasbeenrotated12002-9(b)"..CH3CH3-::NN(a)HHH"NOTINTER"H-C-C=N-"C-H,CCONVERTIBLEC.......HCH3",.........HCH3",../,�I,"-.3H2HsptwoCH3"sonoppositetwoCH3"sonthesamesp课后答案网sidesoftheC=NsideoftheC=N(c)theCH3ontheNisonthesameside:N"..CH3asanotherCH3nomatterhowitisdrawn-onlyonepossiblestructure"CH3",..C"CH32-10HF"/(a)andwww.hackshp.cnC=C/"-FHtrans(c)(b)nonoci.s-trans,iSO.meri.SmtwoId.entIcal.groupsononecis.-tramIs.omen.smcaronbfthdblbd(d)no0eoueoncis-transIsomensm}(e)Q...CH3H"cis"and"trans"not...C=C,andQC...�c"-,definedforthisexampleHHHCH3CIStrans29
2-11Modelswillbehelpfulhere.(a)cis-transisomers-thefirstistrans,thesecondiscis(b)constitutionalisomers-thecarbonskeletonisdifferent(c)constitutionalisomers-thebrominesareondifferentcarbonsinthefirststructure,onthesamecarboninthesecondstructure(d)samecompound-justflippedover(e)samecompound-justrotated(f)samecompound-justrotated(g)notisomers-differentmolecularformulas(h)constitutionalisomers-thedoublebondhaschangedposition(i)samecompound-justreversed(j)constitutionalisomers-theCH3groupsareindifferentrelativepositions(k)constitutionalisomers-thedoublebondisinadifferentpositionrelativetotheCH32-12(a)2.4D=4.8x8x1.21A8=0.41,or41%ofapositivechargeoncarbonand41%ofanegativechargeonoxygen(b):0:I.........C.......R.....+RBResonanceformAmustbethemajorcontributor.IfBwerethemajorcontributor,thevalueofthechargeseparationwouldbebetween0.5and1.0.EventhoughBis"minor",itisquitesignificant,explaininginpartthehighpolarityoftheC=O.2-13BothNH3andNF3haveapairofnonbondingelectronsonthenitrogen.InNH3,thedirectionofpolarizationoftheN-Hbondsistowardthenitrogen;thus,allthreebondpolaritiesandthelonepairpolarityreinforceeachother.InNF3,ontheotherhand,thedirectionofpolarizationoftheN-Fbondsisawayfromthenitrogen;thethree课后答案网bondpolaritiescancelthelonepairpolarity,sothenetresultisaverysmallmoleculardipolemoment.polaritiesreinforce;polaritiesoppose;largedipolemoment1O·smalldipolemoment�N�H....www.hackshp.cn\"HH2-14Somemagnitudesofdipolemomentsaredifficulttopredict;however,thedirectionofthedipoleshouldbestraightforward,inmostcases.Actualvaluesofmoleculardipolemomentsaregiveninparentheses.(Eachhalogenatomhasthreenonbondedelectronpairs,notshownbelow.)TheC-Hisusuallyconsiderednon-polar.(a)LCi(b)HHit"/H�,+-H�C�largedipole(1.54)C-Flargedipole(1.81)/XX:CIHInet..Inet..30
2-14continued(c)LF(d)�/"cnetdipole=01��largedipole(1.70)�Fneteachendoxygenhas(e)Q1one-halfnegative(f)��0�,o.�0TcomchargepositaseitofistwotheH-CNCD"x�o""o�resonanceforms;seeI..netsolutionto1-38(b)netlargedipole(2.95)roorsmalldipole(0.52)(g)(h)0;13largedipolelargedipole(2.72)�1C1H/"CH3netnet(i)1)1smalldipole(0.67)//N,�netnetH3C,/,""CH3CH3课后答案网largedipole(l.45)(k)F(I)----+�(m)\�CI-Be-CItt��N""Il-Fnetdipole=0H"""\"HFwww.hackshp.cnHnetdipole=0netdipole=0In(k)through(m),thesymmetryofthemoleculeallowstheindividualbonddipolestocancel.2-15Withchlorinesonthesamesideofthedoublebond,thebonddipolemomentsreinforceeachother,resultinginalargenetdipole.Withchlorinesonoppositesidesofthedoublebond,thebonddipolemomentsexactlycanceleachother,resultinginazeronetdipole.C��CIlargenetdipoleC==C1netdipole=0/""HH31
(hydrogenbondsshownaswavybond)2-17(a)(CH3hCHCH2CH2CH(CH3hhaslessbranchingandboilsatahighertemperaturethan(CH3hCC(CH3h.(b)CH3(CH2)sCH20HcanformhydrogenbondsandwillboilatamuchhighertemperaturethanCH3(CH2)6CH3whichcannotformhydrogenbonds.(c)HOCH2(CH2)4CH20Hcanformhydrogenbondsatbothendsandhasnobranching;itwillboilatamuchhighertemperaturethan(CH3hCCH(OH)CH3.Cd)(CH3CH2CH2hNHhasanN-Hbondandcanformhydrogenbonds;itwillboilatahighertemperaturethan(CH3CH2hNwhichcannotformhydrogenbonds.(e)Thesecondcompoundshown(B)hasthehigherboilingpointfortworeasons:BhasahighermolecularweightthanA;andB,aprimaryaminewithtwoN-Hbonds,hasmoreopportunityforforminghydrogenbondsthanA,asecondaryaminewithonlyoneN-Hbond.课后答案网2-18(a)CH3CH20CH2CH3canformhydrogenbondswithwaterandismoresolublethanCH3CH2CH2CH2CH3whichcannotformhydrogenbondswithwater.(b)CH3CH2NHCH3ismorewatersolublebecauseitcanformhydrogenbonds;CH3CH2CH2CH3cannotformhydrogenbonds.www.hackshp.cn(c)CH3CH20Hismoresolubleinwater.ThepolarO-Hgroupformshydrogenbondswithwater,overcomingtheresistanceofthenon-polarCH3CH2grouptowardenteringthewater.InCH3CH2CH2CH20H,however,thehydrogenbondingfromonlyoneOHgroupcannotcarryafour-carbonchainintothewater;thissubstanceisonlyslightlysolubleinwater.(d)Bothcompoundsformhydrogenbondswithwateratthedouble-bondedoxygen,butonlythesmallermolecule(CH3COCH3)dissolves.Thecycliccompoundhastoomanynon-polarCH2groupstodissolve.32
2-19HHHHHHHHHHHHIIIIIIIIIIII(a)H-C-C-C-C-C-H(b)H-C-C=C-C-C-H(c)H-C-C::C-C-C-C-HIIIIIIIIIIIIIIHHHHHHHHHHHHHHalkanealkenealkyne(Usually,weusethetenn"alkane"onlywhennoothergroupsarepresent.)HHHHHHHHHIIIIIIIII(d)H-C-C=C-C-H(e)H-C-C-C-C-H(f)H,C..C=C-HIIC�"C�IIII"IHHH-CC-HH-C-C"CC""/IIHH,"C��"HHC-CHHHIIIH""HHHHcycloalkynecyc10alkanearomatichydrocarbonandalkeneHHHHI;IIIIIHHHHH(g)H,CC-C-C-H(h)H,"HC..,I(i)HC"c",HH-C�"C�IIIH-C�C-C::C-C-H"Co:."C�"C-HIIIIIIIIIHCHH-C...CIH-CC..HHCCCC�IH"H•HH•"""C�...HH""c�"C�_,HHIHIIHC=C-HHIIHHHHcycloalkenealkyne,alkene,cycloalkanearomatichydrocarbonandcycloalkene2-20HHH0HH0HH0HHII"IIIII"II(a)H-C-C-C-H(b)H-C-C-C-C-H(c)H-C-C-C-C-HII课后答案网IIIIIIIHHHHHHHHHaldehydealcoholketoneHHHHIIIIH"H0(d)H-C-C-O-C-C-H(e)H,C..,H(f)H0HIIIIH-C�C-C-O-H"H-"C�"C"-HHHHHwww.hackshp.cnIIIIetherH-C,..C-H.H-CC-HH,.c�HH,...,.c�.HHHHHcarboxylicacidetheroHH"C-H(g)(h)H""C,/H"c;�.H.C-C.HIIHHHketonealdehydealcohol33
2-21HH0HHHHHHH0H11111111111111(a)H-C-C-C-N-C-H(b)H-C-C-N-C-C-H�)H-C-C-C-O-C-H111111111111HHHHHHHHHHCH"amideamineH"HHesterHHHHHHHIIII111(e)H-C-C-O-C-C-H(f)H-C-C-C-C::NII1I111HHHHHHHethernitriIeHI;IHHH,,H(g)H"C"HH0(i)U)H"C.1IIIIIIH-CN-C-HH-C-C-C-C-C-O-HI1"HIIIIH-C,.......C-H.H"CHH"H"CHHHHHHcarboxylicacidcyclicesterketoneandetheramineoa11HH,,H0H(k)H"...C..I(I)HC111(m)HCHH-CN-C-H"H-"c".N-C-C-HH-"C""C"-HI1I11I1HH-C,.......C-H,H-C,.......C-H.HH-CC-HH"CHHC"""HH.�"HHH课后答案网HHH-C-H1cyclicamideamideHketoneandamineHH,.(n)(0)H"CHwww.hackshp.cnH-C"C-C::NI1I1H-C,.......C-H,H-C,""C-H,H"CHH"CHHHHHcyclicesternitrileketone2-22Whentheidentityofafunctionalgroupdependsonseveralatoms,allofthoseatomsshouldbecircled.Forexample,anetherisanoxygenbetweentwocarbons,sotheoxygenandbothcarbonsshouldbecircled.Aketoneisacarbonylgroupbetweentwoothercarbons,soallthoseatomsshouldbecircled.(a)€2§9HCH3(b)€0Y32(gIl(c)CH3C-Halkeneetheraldehyde34
2-22continued(e)CH6c:3)IIH(f)R~C-O-HIIamine(d)HC-NH�carboxylicacidgSuggestedbystudentRichardKing:Risthesymbolthatorganicchemistsusetorepresentalkylandarylgroups.Asyouwillseeinthecourseofyourstudy,thereareamidequiteafewwaysthatcarbonandhydrogenatomscangotogethertoformalkyland(thisalsolookslikeanaldehyde,butarylgroups.Sowhenyouseethissymbol,youshouldknowthatitrepresentsONl..Yanamidehashigher"priority"asyousomecombinationofcarbonandhydrogenatoms-exceptwhenitincludesotherwillseelater)atoms.(g)(h)(i)8aromaticalkeneketone(j)(k)(aromaticCH3CH3�R2-23Pleaserefertosolution1-20,课后答案网page12ofthisSolutionsManual.2-24Theexamplesherearerepresentative.Yourexamplesmaybedifferentandstillcorrect.Whatisimportantinthisproblemistohavethesamefunctionalgroup.(a)alkane:hydrocarbon(b)alkene:containsa(c)alkyne:containsawithallsinglebonds;canbecarbon-carbondoublebondcarbon-carbontriplebondacyclic(noring)orcyclicwww.hackshp.cnHHHHHIIIIIH-C-C-C-HH-C-C==C-HH-C-C-C-HIIIIIIIHHHHHHH(d)alcohol:containsan(e)ether:containsan(f)ketone:conatinsacarbonylOHgrouponacarbonoxygenbetweentwocarbonsgroupbetweentwocarbonsHHHHH0HIIIIIIIIH-C-C-O-HH-C-O-C-HH-C-C-C-HIIIIIIHHHHHH35
2-24continued(g)aldehyde:contains(h)aromatichydrocarbon:(i)carboxylicacid:containsaacarbonylgroupwithaacyclichydrocarbonwithcarbonylgroupwithanOHhydrogenononesidealternatingdoubleandsinglegroupononesideHHabondsH0IIIIIIIH-C-C-C-HH-C-C-O-HIIoIHHH(j)ester:containsacarbonyl(k)amine:containsanitrogen(I)amide:containsacarbonylgroupwithanO-Cononesidebondedtoone,two,orthreegroupwithanitrogenononesidecarbonsHaHHHorRgroupHaHI1/IIIIIIIH-C-C-O-C-HH-C-N-HorRgroupH-C-C-N-HIIIIHHHH(m)nitrile:containsthecarbon-nitrogentriplebond:H3C-CN2-25ModelsshowthatthetetrahedralgeometryofCH2CI2precludesstereoisomers.2-26HH"/3(a)C(b)Cyclopropanemusthave60°bondanglescomparedwiththeusualspH-I_-Hbondangleof109.5°inanacyclicmolecule.CC/HH(c)Likeabentspring,bondsthatdeviatefromtheirnormalanglesorpositionsarehighlystrained.Cyclopropaneisreactivebecausebreakingtheringrelievesthestrain.2-27(a)f)课后答案网(b)0_(c)(J�/H+....../0"""",N--NHH""HI�H?:}"0H�Sp3,nobondanglebecausebothsp3,all==109°www.hackshp.cnoxygenisbondedtoonlyoneatomH(d)/"HbehindtheplaneH"::,CofthepaperH""��)/C�J".C-H",HHJ".HH3allsp,all==109°3anglesaroundspatoms==109°2anglesaroundspcarbon==120°36
2-27continuedinfrontofthebehindtheplane(h)planeoftheofthepape;(i)paper...-/�(j{)aH/""",,�HHCIH3anglesaroundspatom�109°2anglesaroundspatoms�120°2-28Forclarityinthesepictures,bondsbetweenhydrogenandansp3atomarenotlabeled;thesebondsare3s-spoverlap.(b)I;;"..H-C-C-O-HA(A/··109°(e)课后答案网www.hackshp.cn22(f)g()7(�Yfp:�H/I-tC-t1"-H109°HH109°sp3_sp2sp2_sp337
322sp_spsp-s(i)�OJ�IH�-f""<;o,,�C�120°sp3_sp3___I109°II2-sp2+p-p---spH/C�./C�120°CH�/,,�H"HSp2-S2_3spsp2-29Thesecondresonanceformofformamideisaminorbutsignificantresonancecontributor.Itshows2thatthenitrogen-carbonbondhassomedoublebondcharacter,requiringthatthenitrogenbesphybridizedwithbondanglesapproaching120°.°°°0°sp2°:0:1°II+/H-C-N-HH-C=N-HIIHH2-30(a)Themajorresonancecontributorshowsacarbon-carbondoublebond,suggestingthatbothcarbonsare23sphybridizedwithtrigonalplanargeometry.TheCH3carbonissphybridizedwithtetrahedralgeometry.?�2�:R:�:SpH-C-C-C-H.....I-----;.�H-C-C=C-HIIsp3/1IHHHH�norm�or(b)Themajorresonancecontributorshowsacarbon-nitrogendoublebond,suggestingthatallthree2carbonsandthenitrogenaresphybridizedwithtrigonalplanargeometry.+课后答案网••++H-N-C=C-C-H....H-N-C-C=C-H..•H-N=C-C=C-HIIIIIIIIIIIIHHHHHHHHHHHHminorminormajor2-31In(c)and(d),theunshadowedporbitalsareverticalandparallel.Theshadowedporbitalsareperpendicula;andhorizontal.www.hackshp.cn(aJH3C,,,Q2(bJ�Vhn.KNH3C"-M�(d)_QOYoQ?oCH3O[Q[JriJJ
2-32(a)(b)Thecoplanaratomsinthestructurestotheleftandbelowaremarkedwithasterisks.cis(c)(d),H""QD,,,eH,CH],*�C-C,*transHJCMHTherearestillsixcoplanaratoms.2-33Collinearatomsaremarkedwithasterisks.2-34(a)nocis-transisomerism(b)(c)nocis-transisomerism课后答案网(d)Theoretically,cyclopentenecouldshowcis-transisomerism.Inreality,thetransformistoounstabletoexistbecauseofthenece0ssityofstretchedIf/bondsanddeformedbondangles.trans-CycJopentenehasneverbeendetected·.cls"trans"--notpossiblebecauseofringstrain/Jwww.hackshp.cnV(e)thesearecis-transisomers,butthedesignationofcisandtranstospecificstructuresisnotdefinedbecauseoffourdifferentgroupsonthedoublebond39
2-35(a)constitutionalisomers-thecarbonskeletonsaredifferent(b)constitutionalisomers-thepositionofthechlorineatomhaschanged(c)cis-transisomers-thefirstiscis,thesecondistrans(d)constitutionalisomers-thecarbonskeletonsaredifferent(e)cis-transisomers-thefirstistrans,thesecondiscis(f)samecompound-rotationofthefirststructuregivesthesecond(g)cis-transisomers-thefirstiscis,thesecondistrans(h)constitutionalisomers-thepositionofthedoublebondrelativetotheketonehaschanged(whileitistruethatthefirstdoublebondiscisandthesecondistrans,inordertohavecis-transisomers,therestofthestructuremustbeidentical)2-36CO2islinear;itsbonddipolescancel,soithasnonetdipole.S02isbent,soitsbonddipolesdonotcancel...O==C==Ot:o..��"o:....netdipolemoment=0netdipolemoment2-37Somemagnitudesofdipolemomentsaredifficulttopredict;however,thedirectionofthedipoleshouldbestraightforwardinmostcases.Actualvaluesofmoleculardipolemomentsaregiveninparentheses.(TheC-Hbondisusuallyconsiderednon-polar.)(a)�"x"--J/............CH3NU1or/H/"CH3netnetlargedipolemomentlargedipolemoment��课后答案网�/BrBrIllI/II0;/0(b)CH3-CNCJ(c)C(d)B��I�BrR11netCnetdipolemoment=0netlargedipolemoment(3.96)CH3/"CH3electronpairsonbrominesarenotshownwww.hackshp.cnlargedipolemoment(2.89)(04N""�b,(g)CH2,�/CH/CI;"C/22netI11/IICH2/C""netCH2CH2"CH"C/2HH2moderatedipolemomentmoderatedipolemomentelectronpairsonchlorinearenotshownnetdipolemoment=040
2-38DiethyletherandI-butanoleachhaveoneoxygen,soeachcanformhydrogenbondswithwater(watersuppliestheHforhydrogenbondingwithdiethylether);theirwatersolubilitiesshouldbesimilar.TheboilingpointofI-butanolismuchhigherbecausethesemoleculescanhydrogenbondwitheachother,thusrequiringmoreenergytoseparateonemoleculefromanother.Diethylethermoleculescannothydrogenbondwitheachother,soitisrelativelyeasytoseparatethem.CH3CH2-0-CH2CH3CH3CH2CH2CH2-OHdiethyletherI-butanolcanhydrogenbondwithwatercanhydrogenbondwithwatercannothydrogenbondwithitselfcanhydrogenbondwithitself2-39o-CN-CH]OHN-methyIpyrroIidinepiperidinetetrahydropyrancyc!opentanolb.p.81°Cb.p.106DCb.p.88DCb.p.141°C(a)PiperidinehasanN-Hbond,soitcanhydrogenbondwithothermoleculesofitself.N-MethylpyrrolidinehasnoN-H,soitcannothydrogenbondandwillrequirelessenergy(lowerboilingpoint)toseparateonemoleculefromanother.(b)Twoeffectsneedtobeexplained:1)Whydoescyclopentanolhaveahigherboilingpointthantetrahydropyran?and2)Whydotheoxygencompoundshaveagreaterdifferenceinboilingpointsthantheanalogousnitrogencompounds?Theanswertothefirstquestionisthesameasin(a):cyclopentanolcanhydrogenbondwithitsneighborswhiletetrahydropyrancannot.Theanswertothesecondquestionliesinthetext,Table2-1,thatshowsthebonddipolemomentsforC-OandH-OaremuchgreaterthanC-NandH-N;bondstooxygenaremorepolarized,withgreaterchargeseparationthanbondstonitrogen.Howisthisreflectedinthedata?Theboilingpointsoftetrahydropyran(88DC)andN-methylpyrrolidine(81°C)areclose;tetrahydropyranmoleculeswouldhaveaslightlystrongerdipoledipoleattraction,andtetrahydr课后答案网opyranisalittleless"branched"thanN-methylpyrrolidine,soitisreasonablethattetrahydropyranboilsataslightlyhighertemperature.Thelargedifferencecomeswhencomparingtheboilingpointsofcyclopentanol(141DC)andpiperidine(106°C).ThegreaterpolarityofO-HversusN-Hisreflectedinamorenegativeoxygen(moreelectronegativethannitrogen)andamorepositivehydrogen,resultinginamuchstrongerintermolecularattraction.TheconclusionisthathydrogenbondingduetoO-HismuchstrongerthanthatduetoN-H.2-40(a)canhydrogenbondwithitselfwww.hackshp.cnandwithwater(g)canhydrogenbondonlywithwater(b)canhydrogenbondonlywithwater(h)canhydrogenbondwithitselfandwithwater(c)canhydrogenbondwithitselfandwithwater(i)canhydrogenbondonlywithwater(d)canhydrogenbondonlywithwater(j)canhydrogenbondonlywithwater(e)cannothydrogenbond(k)canhydrogenbondonlywithwater(f)cannothydrogenbond(I)canhydrogenbondwithitselfandwithwater2-41Higher-boilingcompoundsarelisted.(a)CH3CH(OH)CH3canformhydrogenbondswithotheridenticalmolecules(b)CH3CH2CH2CH2CH3hasahighermolecularweightthanCH3CH2CH2CH3(c)CH3CH2CH2CH2CH3haslessbranchingthan(CH3hCHCH2CH3(d)CH3CH2CH2CH2CH2ClhasahighermolecularweightANDdipole-dipoleinteractioncomparedwithCH3CH2CH2CH2CH341
2-42(a)(b)etheretheralkenealdehyde(c)(d)ketonearomatic(e)(f)ester(cyclic)amide(cyclic)alkene(g)(h)课后答案网ammeester2-43..:0:www.hackshp.cn:0::0:II"I+/C"//S.............f----J....//S.......CH3tCH3CH3••CH3CH3••CH32t3sp-planarsp-tetrahedralThekeytothisproblemisunderstandingthatsulfurhasalonepairofelectrons.Thesecondresonance3formshowsfourpairsofelectronsaroundthesulfuratom,anelectronicconfigurationrequiringsp2hybridization.SulfurinDMSOcannotbesplikecarboninacetone,sowewouldexpectsulfur"sgeometrytobepyramidal(thefourelectronpairsaroundsulfurrequiretetrahedralgeometry,butthethreeatomsaroundsulfurdefineitsshapeaspyramidal).42
2-44CQ)([)(a)penicillinGamidethioether("thio"meansII\IIsulfurreplacesoxygen)"1_CH2C-N__aromatic(b)dopaminearomatic(Inlaterchapters,youwilllearnthattheOHgrouponabenzeneringisaspecial)©H2functionalgroupcalleda"phenol".ForamIne�Inow,itfitsthebroaddefinitionofanCH2CH2alcohol.)(c)thyroxinearomatic).amme�CH2@ecarboxylicacid课后答案网aromatic(d)testosteronewww.hackshp.cn43'
您可能关注的文档
- MATLAB7.0基础教程 (孙祥 徐流美 吴清 著) 清华大学出版社 课后答案
- 信息安全数学基础 (裴定一 徐祥 著) 人民邮电出版社 课后答案
- matlab程序设计教程 第二版 (刘卫国 著) 中国水利水电出版社 课后答案
- 信息安全数学基础 (裴定一 徐祥 著) 人民邮电出版社 课后答案
- MATLAB基础与应用教程 (蔡旭辉 刘卫国 著) 人民邮电出版社 课后答案
- 信息安全数学基础 (许春香 著) 电子科技大学出版社 课后答案
- MPA&MBA研究生英语教程 第一版 (陶伟 著) 中国科学技术大学出版社 课后答案
- mpc (manufacturing 著) 广东工业大学机电工程系 课后答案
- 计算机操作系统课后习题答案(第四版)
- 计算机网络(第六版)谢希仁著课后习题答案
- P28 第二章 会计要素与会计等式
- 信息光学(教材+详细答案 二合一)苏显渝 李继陶
- P55 第三章 账户与复式记账
- P158第六章 主要经济业务核算
- 计算机网络第六版谢希仁编著课后习题答案
- 信息理论基础 (周荫清 著) 北京航空航天大学出版社 课后答案
- Pattern recognition and machine learning (Christopher M. Bishop 著) Springe
- 信息理论与编码 第二版 (吕锋 王虹 著) 人民邮电出版社 课后答案
相关文档
- 施工规范CECS140-2002给水排水工程埋地管芯缠丝预应力混凝土管和预应力钢筒混凝土管管道结构设计规程
- 施工规范CECS141-2002给水排水工程埋地钢管管道结构设计规程
- 施工规范CECS142-2002给水排水工程埋地铸铁管管道结构设计规程
- 施工规范CECS143-2002给水排水工程埋地预制混凝土圆形管管道结构设计规程
- 施工规范CECS145-2002给水排水工程埋地矩形管管道结构设计规程
- 施工规范CECS190-2005给水排水工程埋地玻璃纤维增强塑料夹砂管管道结构设计规程
- cecs 140:2002 给水排水工程埋地管芯缠丝预应力混凝土管和预应力钢筒混凝土管管道结构设计规程(含条文说明)
- cecs 141:2002 给水排水工程埋地钢管管道结构设计规程 条文说明
- cecs 140:2002 给水排水工程埋地管芯缠丝预应力混凝土管和预应力钢筒混凝土管管道结构设计规程 条文说明
- cecs 142:2002 给水排水工程埋地铸铁管管道结构设计规程 条文说明