- 101.66 KB
- 2022-04-22 13:41:54 发布
- 1、本文档共5页,可阅读全部内容。
- 2、本文档内容版权归属内容提供方,所产生的收益全部归内容提供方所有。如果您对本文有版权争议,可选择认领,认领后既往收益都归您。
- 3、本文档由用户上传,本站不保证质量和数量令人满意,可能有诸多瑕疵,付费之前,请仔细先通过免费阅读内容等途径辨别内容交易风险。如存在严重挂羊头卖狗肉之情形,可联系本站下载客服投诉处理。
- 文档侵权举报电话:19940600175。
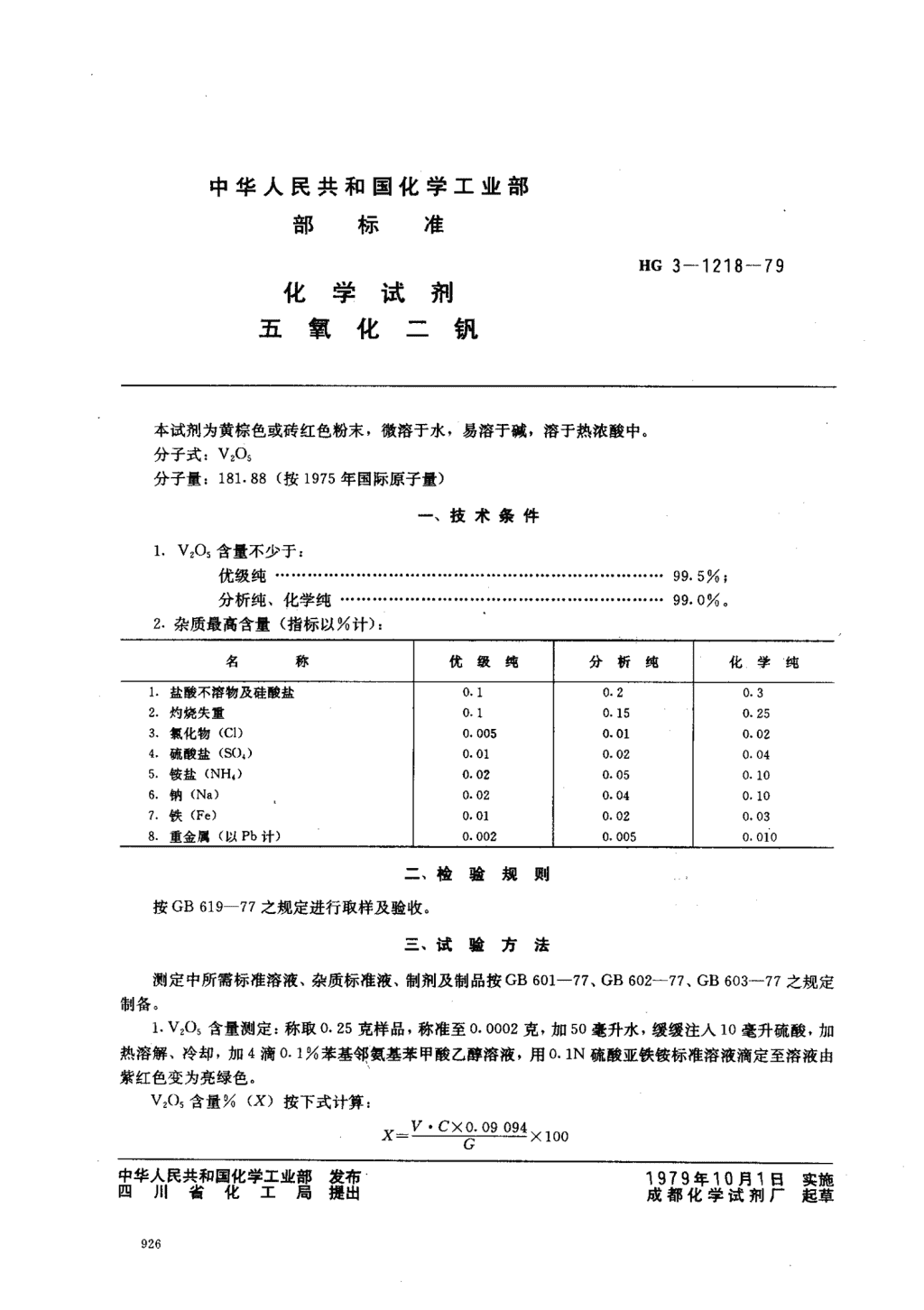
'中华人民共和国化学工业部部标准HG3一1218一79化学试剂五氧化二钒本试剂为黄棕色或砖红色粉末,微溶于水,易溶于碱,溶于热浓酸中。分子式:V205分子量:181.88(按1975年国际原子量)一、技术条件1.VSO:含量不少于:优级纯·····························································⋯⋯99.5%;分析纯、化学纯·····························..·..·................⋯⋯99.0%,2.杂质最高含量(指标以00计):名称优级纯分析纯化学纯1.盐酸不溶物及硅酸盐0.10.20.32.灼烧失重0.10.150.253.抓化物(CD0.0050.010.024.硫酸盐(S(),)0.010.020.045.钱盐(NH4)0.020.050.106.钠(Na)0.020.040.107.铁(Fe)0.010.020.038.重金属(以Pb计)0.0020.0050.010二、检验规则按GB619-77之规定进行取样及验收。三、试验方法测定中所需标准溶液、杂质标准液、制剂及制品按GB601-77,GB602-77,GB603-77之规定制备。1.V205含量测定:称取。.25克样品,称准至0.0002克,加50毫升水,缓缓注人10毫升硫酸,加热溶解、冷却,加4滴0.1写苯基邻氨基苯甲酸乙醉溶液,用。.1N硫酸亚铁按标准溶液滴定至溶液由紫红色变为亮绿色。V20,含量%(X)按下式计算:X=竺丝逐全丝卫旦盛X100几J中华人民共和国化学工业部1979年10月1日实施四川省化工局成都化学试剂厂起草
He3一1218一79式中:v硫酸亚铁按标准溶液之用量,毫升;C一硫酸亚铁钱标准溶液之当量浓度,N;G一样品f(量,克;。.09099每毫克当量VP。之克数。2·杂质测定:样品须称准至。.01克。(1)盐酸不溶物及硅酸盐:称取3克样品,加20毫升盐酸及1克盐酸经胺,加热溶解,在水浴上蒸干冷却,加10毫升盐酸、1克盐酸经胺及100毫升水,加热至沸,用无灰滤纸过滤,以含有10yo盐酸的热水洗涤滤渣至洗液无色。将滤渣置于已恒重的增涡中,缓缓加热炭化,于800"C,灼烧至恒重,残渣重量不得大于:优级纯···············-·,·····································甲,···一3毫克;分析纯·····················,·············44,,·········。········,······⋯⋯6毫克;化学纯·.......................................................................9毫克。(2)灼烧失重:称取2克样品,置于恒重的增竭中,称准至。.0002克,于400℃灼烧至恒重。由减轻之重量计算灼烧失重百分数(3)氯化物:量取20毫升“溶液1",稀释至25毫升,加2毫升5N硝酸及1毫升。.1N硝酸银,摇匀,放置10分钟所呈浊度不得大于标准。标准是取下列数量的C1;优级纯·。,·,············‘···,,,················,············,·····,··,·。⋯0.01毫克;分析纯···,································,····.....................。.02毫克;化学纯··········.........................................................。.04毫克。稀释至25毫升,与同体积样品溶液同时同样处理。注:‘溶液1”的制备—称取1克样品,加15毫升10y,氢氧化钠溶液.加热溶解,加25毫升水,10毫升冰乙酸,水浴加热至溶液无色(1^2小时),搅拌〔必要时补充水),冷却,过撼,洗涤.稀释至100毫升(4)硫酸盐:量取10毫升“溶液1=,加5毫升95%乙醇,1毫升3N盐酸,在不断振播下滴加3毫升25写氯化钡溶液,摇匀,放置10分钟。所呈浊度不得大于标准。标准是取下列数量的S04优级纯················。····,,,····································⋯⋯。.01毫克;分析纯-甲······,···········-·,········,·,.·········甲·····⋯。.02毫克;化学纯···················,········································⋯’⋯。04毫克。稀释至10毫升,与同体积样品溶液同时同样处理。(5)钱盐:称取。.5克样品,置于支管蒸馏瓶中,加80毫升不含氨的水,沿壁加人20毫升10环不含氨的氢氧化钠溶液,加热蒸馏出50毫升液体,用装有50毫升水的100毫升比色管收集。取20毫升,加2毫升纳氏试剂,摇匀。所呈黄色不得深于标准。标准是取下列数量的NH4,优级纯·····················,·················,····················。··⋯。.02毫克;分析纯·····················,,···················。····················⋯⋯0.05毫克;化学纯··········‘··‘···································⋯⋯。.10毫克。与样品同时同样处理。(6)钠:原子吸收分光光度法:仪器条件:光源:钠空心阴极灼一;波长:589皇微来
HG3一1218一79火焰:乙炔一空气。测定方法:称取1克样品,加5毫升盐酸及。.5克盐酸经胺,加热溶解,冷却,稀释至100毫升,取10毫升,共四份。按HG3-1013-76第二章第2条第(2)款之规定测定。(7)铁:a.原子吸收分光光度法仪器条件;光源:铁空心阴极灯;波长:248.3毫微米;火焰:乙炔一空气。测定方法:称取5克样品,加25毫升盐酸及3克盐酸经胺,加热溶解,冷却,稀释至100毫升,取20毫升,共四份。按HG3-1013-76第二章第2条第(2)款之规定测定。b.化学方法:称取1克样品,加20毫升IN氢氧化钠加热溶解、冷却,稀释至100毫升。取10毫升,稀释至20毫升,加2毫升用氨水中和至微碱性的10%磺基水杨酸溶液,于水浴中保温5分钟,加5毫升10写氨水,摇匀。所呈黄色不得深于标准。标准是取2毫升INNaOH及下列数量的Fe:优级纯·······························································⋯⋯0.01毫克;分析纯.....................................................................0.02毫克;化学纯·....................................................................。.03毫克。稀释至20毫升,与同体积样品溶液同时同样处理。(8)重金属:称取。.7克样品,加25毫升10%氢氧化钠溶液,加热溶解,冷却,加4毫升300o酒石酸溶液,冷却,加2毫升新配制的5%硫化钠溶液,放置10分钟。所呈暗色不得深于标准。标准是取0.2克样品及下列数量的Pb:优级纯···························································⋯⋯。.010毫克;分析纯”················································,········⋯⋯。.025毫克;化学纯··························································⋯⋯。.050毫克。与样品同时同样处理四、包装及标志1.包装按HG3-119-64之规定。内包装形式:G-3,GZ3;外包装形式:1-1;包装单位:第3类。2.标志按HG3-119-64之规定。'
您可能关注的文档
- HGT3479-1977化学试剂邻苯二甲酸酐(原HGT3-1107-77).pdf
- HGT3479-2003化学试剂邻苯二甲酸酐.pdf
- HGT3480-2000化学试剂氨基乙酸.pdf
- HGT3481-1999化学试剂4-甲基-2-戊酮(甲基异丁基甲酮).pdf
- HGT3482-1978化学试剂氯化锂(原HGT3-1160-78).pdf
- HGT3482-2003化学试剂氯化锂.pdf
- HGT3483-1978化学试剂四苯硼钠(原HGT3-1164-78).pdf
- HGT3483-2003化学试剂四苯硼钠.pdf
- HGT3484-1999化学试剂标准玻璃乳浊液和澄清度标准.pdf
- HGT3485-2003化学试剂五氧化二钒.pdf
- HGT3486-2000化学试剂乙二胺.pdf
- HGT3487-2000化学试剂磷酸氢二钾.pdf
- HGT3488-1980化学试剂结晶四氯化锡(原HGT3-1286-80).pdf
- HGT3488-2003化学试剂结晶四氯化锡.pdf
- HGT3489-2000化学试剂氯化亚铜.pdf
- HGT3490-1980化学试剂线状氧化铜(原HGT3-1289-80).pdf
- HGT3490-2003化学试剂线状氧化铜.pdf
- HGT3491-1999化学试剂活性炭.pdf
相关文档
- 施工规范CECS140-2002给水排水工程埋地管芯缠丝预应力混凝土管和预应力钢筒混凝土管管道结构设计规程
- 施工规范CECS141-2002给水排水工程埋地钢管管道结构设计规程
- 施工规范CECS142-2002给水排水工程埋地铸铁管管道结构设计规程
- 施工规范CECS143-2002给水排水工程埋地预制混凝土圆形管管道结构设计规程
- 施工规范CECS145-2002给水排水工程埋地矩形管管道结构设计规程
- 施工规范CECS190-2005给水排水工程埋地玻璃纤维增强塑料夹砂管管道结构设计规程
- cecs 140:2002 给水排水工程埋地管芯缠丝预应力混凝土管和预应力钢筒混凝土管管道结构设计规程(含条文说明)
- cecs 141:2002 给水排水工程埋地钢管管道结构设计规程 条文说明
- cecs 140:2002 给水排水工程埋地管芯缠丝预应力混凝土管和预应力钢筒混凝土管管道结构设计规程 条文说明
- cecs 142:2002 给水排水工程埋地铸铁管管道结构设计规程 条文说明