- 1.61 MB
- 2022-04-22 13:42:45 发布
- 1、本文档共5页,可阅读全部内容。
- 2、本文档内容版权归属内容提供方,所产生的收益全部归内容提供方所有。如果您对本文有版权争议,可选择认领,认领后既往收益都归您。
- 3、本文档由用户上传,本站不保证质量和数量令人满意,可能有诸多瑕疵,付费之前,请仔细先通过免费阅读内容等途径辨别内容交易风险。如存在严重挂羊头卖狗肉之情形,可联系本站下载客服投诉处理。
- 文档侵权举报电话:19940600175。
'中国科技论文在线http://www.paper.edu.cnFacilepreparationandperformanceofnovelactivated#carbonbasedonbambooshootshellforsupercapacitorsLUQun,ZHANGYouwei,WANGXianyou,GAOJiao,LIUJia,CHENManfang,**5WANGXingyan(SchoolofChemistry,XiangtanUniversity,Xiangtan411105,China)Abstract:Theporouscarbonmaterialpreparedfromnaturallyabundantbambooshootshellfortheapplicationofsupercapacitorisputforward.Itnotonlymakeswastebiomasstobeprofitable,butalsoprovidesasustainabledevelopmentwayforthepreparationofthenewporouscarbonmaterial.The10physicochemicalandelectrochemicalpropertiesoftheas-preparedporouscarbonsamplesarestudiedindetail.Theresultsshowthattheporouscarbonsamplepreparedat5:1weightratioofKOHto2-1bambooshootshellderivedcarbon(BSSDC)exhibitsaspecificsurfaceareaof~3408mg-1-1(BSSDAC-5).Inaddition,theBSSDAC-5showsahighspecificcapacitanceof308Fgat1Agandhighcapacitanceretentionof97.40%evenafter10000cycles,indicatingitspotentialapplicationfor15high-performancesupercapacitors.Keywords:supercapacitor;bambooshootshell;carbonization;activation;activatedcarbon0IntroductionSupercapacitorsareatypeofenergystoragedevices,withfeaturesintermediatebetween[1]20traditionalcapacitorsandrechargeablebatteries.Theyhavealreadyattractedagreatdealofattentionfrombothindustryandacademia,duetoversatilepropertiesofhighpowerdensity,longcyclelife,rapidcharging/dischargingprocess,superiorratecapability,lowmaintenancecost,etc[2].Nowadays,supercapacitorsarewidelyappliedtomanyfields,suchas,digitalwirelessdevices,[3,4]memoryback-upsystems,hybridelectricvehiclesandsoon.Withthefurtherdevelopmentof25applicationfields,supercapacitorshaveemergedasoneofthemostpromisingenergystoragesystems.Aswellknown,accordingtothechargestoragemechanismsaswellastheelectrodeactivematerialsused,supercapacitorscanbedistinguishedaselectrochemicaldoublelayercapacitors[5,6](EDLCs)andpseudo-capacitors.Carbon-basedmaterial,suchasactivatedcarbon,carbon30aerogels,carbonnanofibers,carbonnanotubes,graphene,carbide-derivedcarbon,andtemplate[7]carbons,havebeenextensivelyinvestigatedfortheapplicationofEDLCs.Duetoitsexcellentpropertiessuchaseasyprocessability,higherabundance,lowercost,highersurfaceareaand[2,8,9]porosity,allkindsofactivatedcarbonmaterialsforEDLCsareespeciallypreferred.Currently,allkindsofactivatedcarbonmaterialsaremainlyproducedfromfossil-fuel35(petroleumandcoal)-basedandwood-basedprecursors.However,thefossil-basedcarbonsourcesarelimitedandnon-renewable,andexcessivedeforestationwillleadtoaseriousenvironmentalproblems,thesetwokindsofrawmaterialsmaynotbethemostappropriatechoicetoprepareactivatedcarbonsforsupercapacitors.Wastebiomasssuchasagriculturalby-products,renewableplantresources,duetoitscheaper,readilyavailable,environmentallyfriendly,renewableand[10,11]40structurallyporous,havebecomemoreattractiveprecursorsforpreparingactivatedcarbons.Now,manyeffortshavetakentodevelopbiomassprecursors-basedactivatedcarbonsaselectrodematerialforsupercapacitors,forexample,nutshells,coconuthusk,woodandcoir.MarínFoundations:theNationalNaturalScienceFoundationofChina(GrantNos.51072173,51272221,51302239),theSpecializedResearchFundfortheDoctoralProgramofHigherEducation(GrantNos.20134301130001and20134301120007).Briefauthorintroduction:LUQun(1993-),female,Mastercandidate,majoringinsupercapacitorCorrespondanceauthor:WANGXianyou(1962-),male,professor,majoringinelectrochemistryandenergymaterials.E-mail:wxianyou@126.com-1-
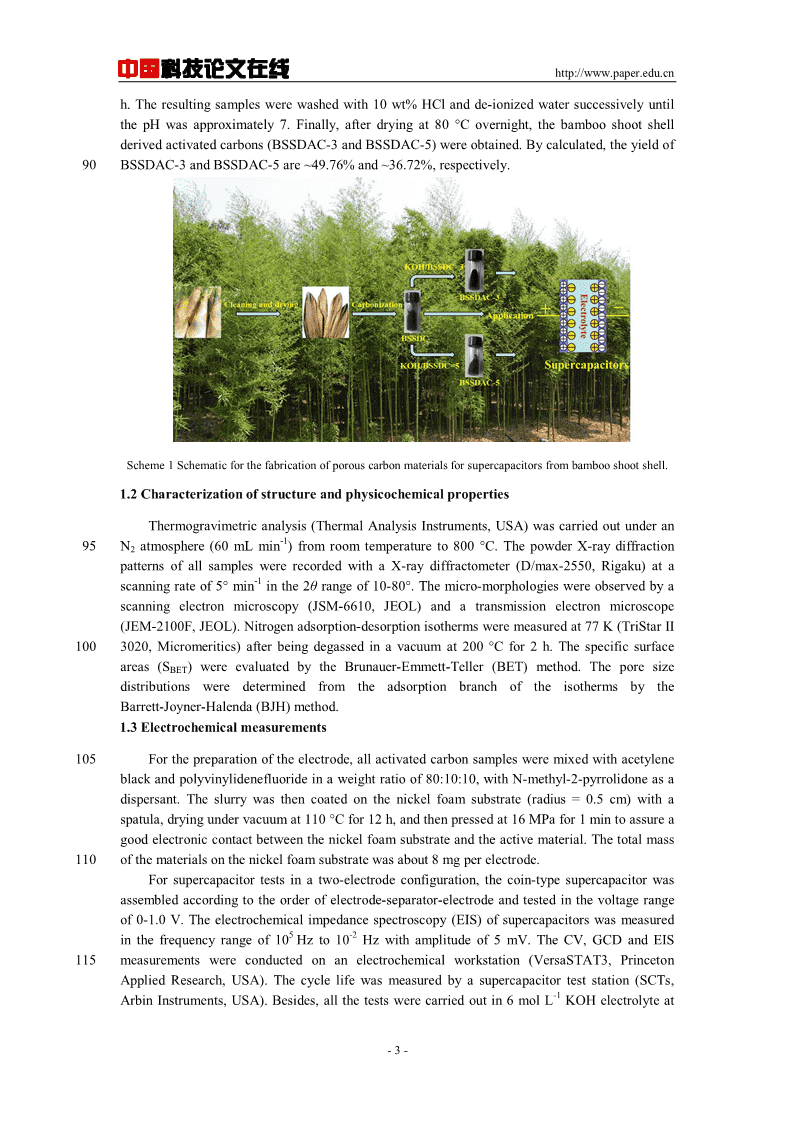
中国科技论文在线http://www.paper.edu.cnetal.preparedactivatedcarbonusingcherrystonesasprecursor,whichexhibitedaspecific-1-2-1-1capacitanceof230Fgat1mAcminthe2molLH2SO4aqueouselectrolyteand120Fgin-1[12]45theaproticmedium1molL(C2H5)4NBF4/acetonitrile.Zhaoetal.obtainedpotato-1-1starch-basedactivatedcarbon,whichpossessedaspecificcapacitanceof314Fgat1Aginthe-1[13]6molLKOHaqueouselectrolyte.Senthilkumaretal.preparedactivatedcarbonfrom-1-1-1sorghumpith,whichexhibitedaspecificcapacitanceof222.1Fgat100mVsinthe1molL[14]H2SO4aqueouselectrolyte.Farmaetal.reportedthepreparationofactivatedcarbonfrom-150fibresofoilpalmemptyfruitbunches,whichobtainedaspecificcapacitanceof149Fgat10-2-1[11]mAcminthe1molLH2SO4aqueouselectrolyte.Inordertopreparetheactivatedcarbonsfortheapplicationofsupercapacitors,although[15-17]manykindsofbiomassprecursorshavebeenreported,whichshowhighspecificcapacitanceandstability,thesearchforlower-costactivatedcarbonsisapriority,andthusthelignocellulosic55wastesasprecursorsalsohavebecomeinterestingandsignificantcandidatesaselectrodematerialsforsupercapacitors.Aswellknown,Chinaisacountryrichinbamboosourcesintheworld,wheretheareaofbambooforestsisveryhuge,evencoveringabout600millionhectares.Therefore,bambooasabundantnaturalbiomassresourceshasbeencurrentlyusedtoprepareactivatedcarbon[18-20]forsupercapacitorsandlithiumionbatteries.However,bambooshootshell(BSS),another60promisingprecursorforactivatedcarbon,hasneveracquiredattentionoftheresearchers.Duringthegrowingprocessofbamboo,thebambooshootshell(BSS)isnaturallypeeledoff,andalargenumberoftheBSSarelefteveryyear.Thus,theBSSisabundantandrenewableresource.Besides,[14]similartoothertypesofwoodenbiomass,theBSSismainlyconsistedofcellulose,hemicelluloseandlignin,suggestingapotentialprecursorcandidateforthepreparationof65activatedcarbon.Moreover,despiteofbeinganinexpensiveandabundantprecursorforproducingactivatedcarbon,BSSderivedactivatedcarbon(BSSDAC)hasnotbeenexplored,nottomentioninanyelectrochemicalapplication.Inthispaper,thesynthesisrouteofactivatedcarbonmaterialsbythedirectcarbonizationofBSSandsubsequentactivationtreatmentwithKOHathightemperatureis70putforward.Thestructure,morphologyandelectrochemicalperformancesoftheas-preparedactivatedcarbonsamplesaselectrodematerialsofsupercapacitorsareinvestigatedindetail.1ExperimentalSection1.1MaterialpreparationAllreagentsareanalyticalgradewithoutfurtherpurification,andthebambooshootshellwas75collectedfromnearbyvillage.Scheme1showstheproceduretosynthesizetheBSSderivedactivatedcarbonmaterials.Atfirst,theBSSwaswashedwithrunningwateranddistilledwatertoensurethatallimpuritieswereremovedanddriedoffintheovenat105°C.Then,thedriedBSSwasfragmentedintosmallpieces(length:~1cm;width:~0.2cm),andthesesmallpieceswere-1pyrolyzedunderargonflowataheatingrampof5°Cmininatubularfurnaceat600°Cfor3h.80Afterthat,thecarbonizedsamplewasimmersedin10wt%HClandboiledfor1htoremovetheimpurityion.Then,thesamplewascentrifuged,rinsedwithwateruntilthepHwasapproximately7,driedat80°Covernight,thebambooshootshellderivedcarbon(BSSDC)wasobtained.Inaddition,theyieldofBSSDCis~29.42%.Then,theas-preparedBSSDCpowderandKOHpelletswithaweightratioof1:3and1:585weregroundfor15minforgooddispersion.Commonly,theactivationprocesswascarriedoutunderArflowbyheatingthemixturesupto400°Cfor0.5h,continuestoheatupto900°Cfor1-2-
中国科技论文在线http://www.paper.edu.cnh.Theresultingsampleswerewashedwith10wt%HClandde-ionizedwatersuccessivelyuntilthepHwasapproximately7.Finally,afterdryingat80°Covernight,thebambooshootshellderivedactivatedcarbons(BSSDAC-3andBSSDAC-5)wereobtained.Bycalculated,theyieldof90BSSDAC-3andBSSDAC-5are~49.76%and~36.72%,respectively.Scheme1Schematicforthefabricationofporouscarbonmaterialsforsupercapacitorsfrombambooshootshell.1.2CharacterizationofstructureandphysicochemicalpropertiesThermogravimetricanalysis(ThermalAnalysisInstruments,USA)wascarriedoutunderan-195N2atmosphere(60mLmin)fromroomtemperatureto800°C.ThepowderX-raydiffractionpatternsofallsampleswererecordedwithaX-raydiffractometer(D/max-2550,Rigaku)ata-1scanningrateof5°mininthe2θrangeof10-80°.Themicro-morphologieswereobservedbyascanningelectronmicroscopy(JSM-6610,JEOL)andatransmissionelectronmicroscope(JEM-2100F,JEOL).Nitrogenadsorption-desorptionisothermsweremeasuredat77K(TriStarII1003020,Micromeritics)afterbeingdegassedinavacuumat200°Cfor2h.Thespecificsurfaceareas(SBET)wereevaluatedbytheBrunauer-Emmett-Teller(BET)method.TheporesizedistributionsweredeterminedfromtheadsorptionbranchoftheisothermsbytheBarrett-Joyner-Halenda(BJH)method.1.3Electrochemicalmeasurements105Forthepreparationoftheelectrode,allactivatedcarbonsamplesweremixedwithacetyleneblackandpolyvinylidenefluorideinaweightratioof80:10:10,withN-methyl-2-pyrrolidoneasadispersant.Theslurrywasthencoatedonthenickelfoamsubstrate(radius=0.5cm)withaspatula,dryingundervacuumat110°Cfor12h,andthenpressedat16MPafor1mintoassureagoodelectroniccontactbetweenthenickelfoamsubstrateandtheactivematerial.Thetotalmass110ofthematerialsonthenickelfoamsubstratewasabout8mgperelectrode.Forsupercapacitortestsinatwo-electrodeconfiguration,thecoin-typesupercapacitorwasassembledaccordingtotheorderofelectrode-separator-electrodeandtestedinthevoltagerangeof0-1.0V.Theelectrochemicalimpedancespectroscopy(EIS)ofsupercapacitorswasmeasured5-2inthefrequencyrangeof10Hzto10Hzwithamplitudeof5mV.TheCV,GCDandEIS115measurementswereconductedonanelectrochemicalworkstation(VersaSTAT3,PrincetonAppliedResearch,USA).Thecyclelifewasmeasuredbyasupercapacitorteststation(SCTs,-1ArbinInstruments,USA).Besides,allthetestswerecarriedoutin6molLKOHelectrolyteat-3-
中国科技论文在线http://www.paper.edu.cnabout25°C.2.Resultsanddiscussion120ThepyrolysisprocessofBSSwasinvestigatedinaThermogravimetricanalyser.Fig.1showsthethermogravimetriccurveofBSSsample.Itclearlyshowsthattheinitialweightdroppedbelow40%oftheoriginalmassataround362°Candmasschangeisrecognizableuntil600°C.Asweallknowthepyrolysisbehaviorofbiomasscanberegardedasthesumpyrolysisprocessof[14]thethreemajorcomponents(hemicellulose,cellulose,andlignin).FromTGcurveinFig.1,it125canbeseenthatthefirstweightloss(~1.87%)engenderedbyheatingthematerialsupto200°Cisthemoistureelimination.Thesecondstageof200-362°C,whichhasamajorweightloss(~59.09%),correspondstoprimarycarbonizationoractivepyrolysis.Thisconsiderablygreaterweightlossmaybeascribedtotheeliminationofvolatilemattersandtarscorrespondingtothe[21]decompositionofhemicellulosesandcelluloses.Thethirdstageinthe362-600°Crange,[22]130whichhasarelativelylittleweightloss(~9.49%),maybelongtothedecompositionoflignin.Above600°C,theweightlosswasverysmallthusindicatingthatthebasicstructureofthecharhasbeenformedapproximatelyatthistemperature.Fig.1TGcurvefortheBSS.135TheXRDpatternsofBSSDC,BSSDAC-3andBSSDAC-5arepresentedinFig.2.Itcanbeobservedaninconspicuoushumpsdiffractionpeaklocatesat2θ=~43°correspondingtoasuperpositionofthegraphitic(100)and(101)planesandabroaddiffractionpeakbetween2θ=20[23]and30°whichisattributedtothegraphitic(002)reflectionforallthesamples.Obviously,thepeaksforBSSDCaremoreintensethanfortheactivatedcarbons.Itindicatesthattheactivation140treatmentisresponsibleforgeneratingtheporousnetworkandopeningupthelayeredgraphitic[24]structure.Besides,asthecontentoftheactivatorKOHisincreasedduringactivation,aremarkabledecreaseinthepeakintensity(002)isobserved,suggestingtheincreaseofdisordered[17]structure.-4-
中国科技论文在线http://www.paper.edu.cn145Fig.2XRDpatternoftheBSSDC,BSSDAC-3andBSSDAC-5.AsshowninFig.3(a)-(c),scanningelectronmicroscopyprovidesinsightsintothemorphologyandsizeofthepreparedcarbonsamples(BSSDC,BSSDAC-3andBSSDAC-5).ItcanbeeasilyobservedinFig.3(a)thatBSSChasatubularshapewiththewidthofabout4μmandrelativelysmoothsurface,suchmorphologyisderivedfromthefiberofBSS;whileinFig.1503(b)-(c),BSSDAC-3andBSSDAC-5generallyinheritthemorphologyandsizeofBSSDCafterKOHtreatmentinargon,indicatingtheretentionofstructure.Besides,thesurfacesofBSSDAC-3andBSSDAC-5becomecoarseandporousduetoreleaseofgasduringtheactivationprocess,andtheBSSDAC-5exhibitshollowinternalstructure.ThemicrostructureofBSSDAC-5isfurtherinvestigatedbyTEM.ItcanbeeasilyobservedinFig.3(d)thatBSSDAC-5revealsabundant155porousstructure.Suchaporousmorphologyandmicrostructuremayprovideaneffectivevolumefortheabsorptionandreservationofelectrolyteionsandbebeneficialtoenablingmoreactive[25,26]sitesforthedouble-layerformationacrosstheinterface.Fig.3SEMimagesof(a)BSSDC,(b)BSSDAC-3and(c)BSSDAC-5;andTEMimageof(d)BSSDAC-5.-5-
中国科技论文在线http://www.paper.edu.cn160TofurtherdeterminethespecificsurfaceareasandporetexturesofthepristineBSSDC,activatedBSSDAC-3andBSSDAC-5,thenitrogenadsorption-desorptionisothermsweremeasured.Fig.4presentsthetypicalnitrogenadsorption-desorptionisothermsandtheBJHporesizedistributionsoftheas-preparedcarbonsamples.AsshowninFig.4(a),theisothermofBSSDCisremarkablydifferentfromthoseofBSSDAC-3andBSSDAC-5afterKOHactivation.165AccordingtotheIUPACclassification,theisothermofBSSDCshowsthetypeIbehavior.Incontrast,thecurvesofBSSDAC-3andBSSDAC-5showakneeandincreasingplateau,whichrevealsanintermediateisothermbetweentypeIandIIandsuggestsacombinationofmicro-andmesoporousstructuresinthepreparedcarbonsamples.Theearlystageoftheisothermswiththesharpincreasecorrespondstomicroporefilling;thesloppyregionatahighrelativepressure[17]170indicatesthemultilayeradsorptiononthemesopores.Inaddition,withincreasingtheamountofactivationagent(KOH),theadsorptioncapacityoftheas-preparedcarbonsamplesalsoincreasewithrelativepressureuntilP/P0≈0.5.Besides,itisgenerallyknownthatanisothermwithaslighthysteresisloopandwidekneesatP/P0ofaround0.35-0.5indicatesthepresenceofa[17]considerableamountofsmallmesopores,whichcanbeobservedinthecurveofBSSDAC-5.175Fig.4(a)Nitrogenadsorption-desorptionisothermsand(b)poresizedistributionplotofBSSDC,BSSDAC-3andBSSDAC-5.Usually,accordingtoIUPACstandardtheporescanbeclassifiedintothreecategories:namely,micropore(<2nm),mesopore(2-50nm)andmacropore(>50nm).Theporesize180distributionsofallthesamplesareillustratedinFig.4(b).TheresultsrevealthattheadditionofKOHsignificantlyaffectstheporesizedistribution.WiththeincreaseoftheweightrationofKOH/BSSDC,theporesizeofthepreparedcarbonsamplesalsoenlarge.ThisphenomenoncanbeexplainedbythepossibilitythatKOHcanreactwithcarbontoreleaseH2,COandCO2,which[10]contributetotheformationofpores.Thetypicalreactionmechanismisconsideredasfollow:6KOH+2C→2K+3H+2KCO223(1)KCO23→KO+CO22(2)185CO+C2→2CO(3)KCO+2C23→2K+3CO(4)KO+C2→2K+CO(5)MoredetailedinformationaboutthetexturalpropertiesofallsamplesislistedinTable1.As2-1showninTable1,themeasuredBETspecificsurfaceareasare589,2723and3408mgwiththe3-1porevolumesare0.25,1.42and2.21cmgforBSSDC,BSSDAC-3andBSSDAC-5,respectively.Obviously,itcanbeseenthatBSSDAC-5exhibitsthehighestspecificsurfacearea190andlargestmicroporevolumeamongallsamples.Besides,asKOHscalesup,moreKOHis-6-
中国科技论文在线http://www.paper.edu.cnbelievedtotakepartintheactivationprocesstoresultinamoreporousstructure,whichleadsto[10,17]anincreaseinthespecificsurfaceareaofthesamplesandenlargementoftheporesizes.Table1Texturalparametersofallthesamplesobtainedfromnitrogenadsorption-desorptionisotherms.SamplesBETsurfaceareaPorevolumeMicroporevolumeBETPoresize2-13-13-1(mg)(cmg)(cmg)(nm)BSSDC5890.250.211.72BSSDAC-327231.421.122.28BSSDAC-534082.211.342.52195ToinvestigatetheelectrochemicalperformanceofsupercapacitorsusingtheBSSDC,-1BSSDAC-3,andBSSDAC-5aselectrodematerials,theCVmeasurementsin6molLKOHelectrolyteareperformedatdifferentscanratesinthevoltagerangeof0-1.0V.AsshowninFig.-15(a),theCVcurvesforallthesamplesatascanrateof10mVsshowanearlyrectangularshape,theresultsuggestsatypicalEDLCcharacteristic.Therefore,theenergy-storagemechanismof200suchsupercapacitorscanbeexplainedbyelectricaldouble-layertheory,whichismainlybasedontheaccumulationofchargecarriersacrosstheelectrode-electrolyteinterface,andsubsequently[17,27]formsthedoublelayer.Inaddition,afterKOHtreatmenttheareasurroundedbyCVcurveisapparentlylargerthanthatofthepristineBSSDC,indicatingthatKOHactivationtreatmentofthesamples(BSSDAC-3andBSSDAC-5)havemuchmorespecificcapacitance,andalsosuggesting205thatBSSDAC-5canobtainthehighestspecificcapacitanceamongallsamples.Furthermore,Fig.5(b)-(d)displaytheCVcurvesofBSSDC,BSSDAC-3andBSSDAC-5atdifferentscanrates-1-1from5mVsto100mVs.AspresentedinFig.5(b)-(d),theCVcurvesoftheBSSDCsupercapacitorshowrelativelyrectangularshapes,butanobviousdistortioncanbeobservedwith-1anincreasingthescanrate,especiallyatthescanrateof100mVs.Onthecontrary,theCV210curvesoftheBSSDAC-3andBSSDAC-5supercapacitorsshowquasi-rectangularshapewithout-1-1obviousdistortionwiththescanrateincreasingfrom5mVsto100mVs,suggestingbetterreversibility,ratecapabilityandsupercapacitivebehaviorsthanthatofthepristineBSSDCsupercapacitor.TheoutstandingelectrochemicalperformanceoftheBSSDAC-5supercapacitermaybeattributedtoitsenlargedspecificsurfaceareaandthepresenceofahigherpercentage215mesopores.-7-
中国科技论文在线http://www.paper.edu.cn-1Fig.5(a)TheCVcurvesforBSSDC,BSSDAC-3andBSSDAC-5supercapacitorsatascanrateof10mVs,CV-1curvesfor(b)BSSDC,(c)BSSDAC-3and(d)BSSDAC-5supercapacitorsatscanratesrangingfrom5mVsto-1100mVs.220Tofurtherestimatetheelectrochemicalperformanceandcomparethesupercapacitivebehavioursofthesamples,theGCDtestsofthesupercapacitorusingdifferentsamplesas-1electrodesareconductedinthevoltagerangeof0-1.0Vatacurrentdensityof1Ag.AsshowninFig.6(a),theGCDcurvesofallthesupercapacitorsshowagenerallysymmetricalshapeandlinearrelationshipwithtime,suggestingthetypicalcapacitivebehaviour.Moreover,thespecific225capacitancesoftheelectrodematerialscanbecalculatedfromthedischargecurvesofsupercapacitorsaccordingtoEq.(6):4It×ΔC=(6)mmVΔ-1whereCmisthegravimetricspecificcapacitanceoftheas-preparedelectrodes(Fg),Iisthedischargecurrent(A),misthetotalmassofactivematerials(g),ΔVisthevoltageof[28]230supercapacitor(V),Δtisthedischargetime(s).Obviously,itcanbeobservedthatthedischargetimeofthepreparedcarbonsamplesbecomelongerandlongeratthesamecharge/dischargeratewithincreasedKOHconcentration,whichtestifiesthatBSSDAC-5exhibitsthehighestspecificcapacitanceamongallsamples.ThroughcalculationbasedontheGCDcurvesinFig.6(a),thespecificcapacitancesofBSSDC,BSSDAC-3,andBSSDAC-5areincreasedto90,-1235265,and308Fg,respectively,whichisconsistentwiththevariationtendencyofspecificsurfacearea.Furthermore,theGCDcurvesofBSSDAC-5supercapacitoratdifferentcurrentdensitiesare-1-1presentedintheFig.6(b).Withincreasingthecurrentdensityfrom1Agto5Ag,theGCDcurvesstillshowagoodsymmetricalshape,suggestingagoodreversibility,highratecapabilityandgoodsupercapacitivebehaviors.-8-
中国科技论文在线http://www.paper.edu.cn240Fig.6TheGCDcurvesfor(a)BSSDC,BSSDAC-3andBSSDAC-5supercapacitorsatthecurrentdensityof1A-1-1-1g,(b)theGCDcurvesforBSSDAC-5supercapacitorsatthecurrentdensitiesrangingfrom1Agto5Ag.and-1(c)specificcapacitancesofBSSDC,BSSDAC-3andBSSDAC-5atdifferentcurrentdensitiesfrom1Agto5A-1g.245Fig.6(c)showsthespecificcapacitancesofBSSDC,BSSDAC-3,andBSSDAC-5samplesatdifferentcurrentdensities.ItcanbefoundthatthespecificcapacitanceofBSSDAC-5isthe-1-1highestamongallsamplesatthesamecurrentdensity,whichisupto308Fgat1Agandstill-1-1remains263Fgatahighcurrentdensityof5Ag(ca.85.40%ofcapacitanceretention),suggestingthattheenhancedspecificsurfaceareabenefitsEDLCformation.Whereas,BSSDC-1-1-1-1250deliversaspecificcapacitanceof90Fgat1Agandremains44Fgat5Ag(ca.71.70%of-1capacitanceretention),andBSSDAC-3deliversaspecificcapacitanceof265Fgandremains-1-1190Fgat5Ag(ca.71.70%ofcapacitanceretention).ThesedemonstratethatthesupercapacitorusingBSSDAC-5aselectrodematerialhasremarkablespecificcapacitanceandexcellentratecapability.Inaddition,thespecificcapacitancesofdifferentbiomass-derived255activatedcarbonsarelistedinTable2.Asshown,allthesamplesexhibitarelativelyhighspecific-1capacitance(>150Fg).Obviously,thespecificcapacitanceofbambooshootshell-derived[29-36]activatedcarbonismuchhigherthanthatofmostotheractivatedcarbons,indicatingabetterelectrochemicalperformance.260Table2Electrochemicalperformanceofsupercapacitorsbasingondifferentbiomass-derivedactivatedcarbons.SamplesSpecificcapacitancesCurrentdensityRef.-1-2Oilpalmemptyfruit149Fg10mAcm11-1-2Cherrystones230Fg1mAcm12-1-1Corncob221Fg0.5Ag29-1-1Shiitakemushroom306Fg1Ag30-9-
中国科技论文在线http://www.paper.edu.cn-1-1Potatowasteresidue255Fg1Ag31-1-1Cotton283Fg1Ag32-1-1Humanhair340Fg1Ag33-1-1Coconutshellgranules246Fg0.25Ag34-1-1Bamboo301Fg0.1Ag35-1-1Waterbamboo268Fg1Ag36-1-1Bambooshootshell308Fg1AgInthisworkInordertoanalyzetheimpedancebehaviorsofthepreparedcarbonmaterials,theelectrochemicalimpedancespectroscopy(EIS)isperformedunderopen-circuitvoltageconditions.Fig.7(a)displaystheNyquistplotsofthesupercapacitorsusingBSSDC,BSSDAC-3and5-2265BSSDAC-5aselectrodematerialsinthefrequencyrangeof10-10Hz.Asshown,theNyquistplotsofallthesupercapacitorsconsistofthreeregions,includingansemicircleathighfrequencyregion,alinewithaslopeabout45°inmiddlefrequencyregionandastraightlineatlowfrequencyregion,indicatingthattheimpedanceresponseisatypicalcapacitivebehaviorofporouscarbonelectrodes.Itiswellknownthatthesemicircleshapedependsontheadsorptionkineticsof270theionsatamicro/mesoporouscarbonelectrode,theseriesresistanceofamaterial,chargetransferresistanceinsidethemacro/mesoporouscarbonstructureandthemasstransferresistance[37,38]inthemicroporouscarbonelectrode(RCE)athigherfrequency.Besides,theequivalentseriesresistance(ESR)valuescanbeobtainedfromx-interceptswiththerealaxisoftheNyquistplotsinhighfrequencypartare1.04Ω,0.46Ωand0.22ΩforBSSDC,BSSDAC-3andBSSDAC-5275supercapacitors,respectively.Inaddition,thesumofESR,RCEandtheinternaldistributionoftheelectrolyteresistancevaluesintheporematrixofcarbonelectrodes(Rpore),i.e.,thetotalpolarizationresistance,isobtainedbyextrapolatingthelow-frequencylinearpartof-Z""vs.Z"plotstoZ""=0.AspresentedintheNyquistplots,thetotalpolarizationresistancesofthesupercapacitorsdecreaseclearlywiththeincreaseoftheKOHconcentration,andtheBSSDAC-5280supercapacitorexhibitsthelowestresistanceamongallthesamples.Furthermore,itcanbeeasilyobservedfromtheinsertinFig.7(a)thattheNyquistplotofBSSDAC-5isnearlyverticallinesatlowfrequencyregion,suggestingthattheelectrodeshowsoutstandingiondiffusionandmigration[39]behaviorandatypicaldouble-layerchargestoragebehavior.Furthermore,thecapacitance-frequencyplotsareshowninFig.7(b).Thespecific285capacitancevaluescanbeestimatedbyC=−1/(2πƒmZ"")(7)-1whereC,f,Z″,andmarethespecificcapacitance(Fg),thefrequency(Hz),theimaginary[40]impedance(Ω)andthetotalmassofactivematerial(g),respectively.AspresentedinFig.7(b),-1itcanbefoundthatthespecificcapacitanceoftheBSSDAC-5electrodeis264Fginthe-1290frequencyregionsof0.01Hz,whichishigherthanthoseofBSSDC(44Fg)andBSSDAC-3-1(198Fg)electrodes.Additionally,thetrendofthespecificcapacitancesfortheas-preparedelectrodesevaluatedfromthelowfrequencydataofthespectraisconsistentwiththeresultsofCVandcharge/dischargetests.Fig.7(c)displaystheBodeplotsforBSSDC,BSSDAC-3andBSSDAC-5supercapacitors.ItcanbeeasilyobservedthatthephaseanglesofBSSDC,295BSSDAC-3andBSSDAC-5supercapacitorsare-57.7,-83.1and-85.3°,respectively.These-10-
中国科技论文在线http://www.paper.edu.cnfurtherdemonstratethegoodcapacitivepropertyofbambooshootshell-derivedactivatedcarbons,especiallytheBSSDAC-5.Moreover,Fig.7(d)showstheevolutionoftheseriescapacitancevalues(Cs)atω→0andtheparallelcapacitance(Cp),andCs,CparecalculatedaccordingtothefollowingEq.(8)and(9):300Cj=1/(ω″)Z(8)s2C=Z/(Z″||ω)(9)p[41]where|Z|istheimpedancemodulus,andωistheangularfrequency.ItcanbeseenfromFig.7(d)thatallthecurvesshowatypicaldropofcapacitancewiththeincreaseofthefrequency.For[42]theideallypolarizationsystem,Cp/Cs=1.Obviously,thevaluesofCp/CsforBSSDC,305BSSDAC-3andBSSDAC-5supercapacitorsinlowfrequencyare~0.709,~0.985and~0.993,respectively.Inaddition,thesmallestdeviationofCp/Csabout0.007from1.0isobservedforBSSDAC-5supercapacitor,indicatinganearlyidealpolarizability.ThroughwholeanalysisEISresults,itcanbefoundthattheimpedancebehaviorofBSSDAC-5issuperiortothatofBSSDCandBSSDAC-3.310Fig.7(a)Nyquistplotswiththeexpandedhigh-frequencyregionoftheplotinset,(b)capacitance-frequencyplots,(c)Bodephaseangleplotsand(d)CP/CSvs.frequencydependenciesoftheBSSDC,BSSDAC-3andBSSDAC-5supercapacitors.Powerdensityandenergydensityareveryimportantparameterstoevaluatethepractical315applicationofsupercapacitors.TheenergydensityandpowerdensityarecalculatedfromtheGCD-1-1testsofsupercapacitorsatthecurrentdensitiesrangingfrom1Agto5AgandaregiveninaRagoneplot(Fig.8),accordingtothefollowingEqs.(10)and(11):2Cm×ΔV1000E=×(10)83600-11-
中国科技论文在线http://www.paper.edu.cn-1whereE,CmandΔVaretheenergydensity(Whkg),thespecificcapacitanceofthecarbons(F-1320g)andthecellvoltage(V),respectively.E×3600Ρ=(11)Δt-1-1whereP,EandΔtarethepowerdensity(Wkg),theenergydensity(Whkg)anddischargetime(s),respectively.Fig.8showstheRagoneplotsofBSSDC,BSSDAC-3andBSSDAC-5supercapacitors.ItcanbeobviouslyseenthatBSSDAC-5supercapacitorachievesthehighest325energydensityatthesamepowerdensityamongallsupercapacitors.Moreover,BSSDAC-5-1supercapacitordeliversthemaximumenergydensityof~10.5Whkg,whereasBSSDCand-1BSSDAC-3supercapacitorspresentenergydensitiesof~3.1and~9.2Whkg,respectively.In-1addition,theBSSDAC-5supercapacitorcanstillmaintainthehighenergydensityof9.1Whkg-1whenthepowerdensityincreasesto2500Wkg.Thehighpowerdensityandenergydensityof2-1330BSSDAC-5aremainlyattributedtothehighspecificsurfacearea(3408mg)withalargepore3-1volume(2.21cmg)andabundantmicroporousstructure,whichprovidelargenumberof[43]reactionsitestoresultintheaccumulationofchargecarriers.-1-1Fig.8Ragoneplotsoftheirsupercapacitorsmeasuredatcurrentdensitiesvaryingfrom1Agto5Ag.335Longcyclelifeofsupercapacitorisoneofthemostimportantparametersforpracticalapplications.AsbeingseeninFig.9,thecyclelifeperformanceofBSSDAC-5supercapacitoris-1-1testedfor10000cyclesatacurrentdensityof1Ag.Itsinitialcapacitanceisupto~308Fg-1andstillremains~300Fgafter10000cycles(ca.97.40%ofcapacitanceretention).Furthermore,thecoulombicefficiencyremainsat100%duringthecyclingprocess.Thus,itisbelievedthatthe340BSSDAC-5supercapacitorobtainsanexcellentlong-termcyclingstability,suggestingthatBSSDAC-5couldbeusedasahigh-performanceelectrodematerialforsupercapacitors.-12-
中国科技论文在线http://www.paper.edu.cn-1Fig.9CycleperformanceforsupercapacitorusingBSSDAC-5aselectrodematerialatacurrentdensityof1Ag.3.Conclusion345Thenovelbambooshootshell-derivedactivatedcarbonhavebeenobtainedat600°Cfor3hinAratmosphereandfollowingactivatedbyKOHat900°Cfor1h.Basedontheexperimentalresults,theBSSDAC-5synthesizedat5:1weightratioofKOHtobambooshootshell-derived-1-1carbonshowsahighspecificcapacitanceof308Fgin6molLKOHelectrolyteatacurrent-1-1-1densityof1Agandstillretainsupto263Fgatacurrentdensityof5Ag.Besides,italso-1350exhibitsexcellentcapacityretentionof97.40%after10000cyclesatacurrentdensityof1Ag.TheoutstandingelectrochemicalperformancesofBSSDAC-5maybeattributedtoahighspecific2-13-1surfacearea(3408mg)withalargeporevolume(2.21cmg)andabundantmicroporosity,whichprovidealargenumberofreactionsitestoresultintheaccumulationofchargecarriersandhighspecificcapacitance.Therefore,BSSAC-5willbeaverypromisingcandidateastheelectrode355materialsforhighperformancesupercapacitors.Allinall,thehighspecificsurfaceareaactivatedcarbonsfortheapplicationofthesupercapacitorcanbepreparedthroughasimpleandeffectivewayfromalow-cost,highlyabundantbiomasssource,thatis,bambooshootshell.Thisnotonlymakeswasteprofitable,butalsoprovidesasustainabledevelopmentrouteforthepreparationofthehighperformanceactivatedcarbonfortheapplicationofthesupercapacitor.360AcknowledgementsThisworkwasfinanciallysupportedbytheNationalNaturalScienceFoundationofChina(GrantNos.51072173,51272221,51302239),theSpecializedResearchFundfortheDoctoralProgramofHigherEducation(GrantNos.20134301130001and20134301120007).365References[1]LargeotC,PortetC,ChmiolaJ,etal.Relationbetweentheionsizeandporesizeforanelectricdouble-layercapacitor[J].JournaloftheAmericanChemicalSociety,2008,130(9):2730-2731.[2]ZHANGLL,ZHAOXS.Carbon-basedmaterialsassupercapacitorelectrodes[J].ChemicalSocietyReviews,2009,38(9):2520-2531.370[3]SimonP,GogotsiY.Materialsforelectrochemicalcapacitors[J].Naturematerials,2008,7(11):845-854.[4]MillerJR,SimonP.Electrochemicalcapacitorsforenergymanagement[J].ScienceMagazine,2008,321(5889):651-652.[5]BurkeA.Ultracapacitors:why,how,andwhereisthetechnology[J].Journalofpowersources,2000,91(1):37-50.375[6]WinterM,BroddRJ.Whatarebatteries,fuelcells,andsupercapacitors?[J].ChemicalReviews,2004,104:4245-4270.-13-
中国科技论文在线http://www.paper.edu.cn[7]BoseS,KuilaT,MishraAK,etal.Carbon-basednanostructuredmaterialsandtheircompositesassupercapacitorelectrodes[J].JournalofMaterialsChemistry,2012,22(3):767-784.[8]FrackowiakE,BeguinF.Carbonmaterialsfortheelectrochemicalstorageofenergyincapacitors[J].Carbon,3802001,39(6):937-950.[9]FrackowiakaE,BéguinF.Carbonmaterialsforelectrochemicalcapacitors[J].Journalofpowersources,2010,195(24):7880-7903.[10]WANGJC,KaskelS.KOHactivationofcarbon-basedmaterialsforenergystorage[J].JournalofMaterialsChemistry,2012,22(45):23710-23725.385[11]FarmaR,DeramanM,AwitdrusA,etal.Preparationofhighlyporousbinderlessactivatedcarbonelectrodesfromfibresofoilpalmemptyfruitbunchesforapplicationinsupercapacitors[J].Bioresourcetechnology,2013,132:254-261.[12]MarínMO,FernándezJA,LázaroMJ,etal.Cherrystonesasprecursorofactivatedcarbonsforsupercapacitors[J].MaterialsChemistryandPhysics,2009,114(1):323-327.390[13]ZHAOS,WANGCY,CHENMM,etal.Potatostarch-basedactivatedcarbonspheresaselectrodematerialforelectrochemicalcapacitor[J].JournalofPhysicsandChemistryofSolids,2009,70(9):1256-1260.[14]SenthilkumarST,SenthilkumarB,BalajiS,etal.Preparationofactivatedcarbonfromsorghumpithanditsstructuralandelectrochemicalproperties[J].MaterialsResearchBulletin,2011,46(3):413-419.[15]CHENWX,ZHANGH,HUANGYQ,etal.Afishscalebasedhierarchicallamellarporouscarbonmaterial395obtainedusinganaturaltemplateforhighperformanceelectrochemicalcapacitors[J].JournalofMaterialsChemistry,2010,20(23):4773-4775.[16]HUANGWT,ZHANGH,HUANGYQ,etal.Hierarchicalporouscarbonobtainedfromanimalboneandevaluationinelectricdouble-layercapacitors[J].Carbon,2011,49(3):838-843.[17]KarthikeyanK,AmareshS,LeeSN,etal.ConstructionofHigh‐Energy‐DensitySupercapacitorsfrom400Pine‐Cone‐DerivedHigh‐Surface‐AreaCarbons[J].ChemSusChem,2014,7(5):1435-1442.[18]JIANGJ,ZHUJH,AIW,etal.Evolutionofdisposablebamboochopsticksintouniformcarbonfibers:asmartstrategytofabricatesustainableanodesforLi-ionbatteries[J].Energy&EnvironmentalScience,2014,7(8):2670-2679.[19]KimYJ,LeeBJ,SuezakiH,etal.Preparationandcharacterizationofbamboo-basedactivatedcarbonsas405electrodematerialsforelectricdoublelayercapacitors[J].Carbon,2006,44(8):1592-1595.[20]ZHOUXH,LILF,DONGSM,etal.Arenewablebamboocarbon/polyanilinecompositeforahigh-performancesupercapacitorelectrodematerial[J].JournalofSolidStateElectrochemistry,2012,16(3):877-882.[21]SricharoenchaikulV,PechyenC,Aht-ongDD,etal.Preparationandcharacterizationofactivatedcarbon410fromthepyrolysisofphysicnut(JatrophacurcasL.)waste[J].Energy&Fuels,2007,22(1):31-37.[22]RuffordTE,JurcakovaDH,KhoslaK,etal.Microstructureandelectrochemicaldouble-layercapacitanceofcarbonelectrodespreparedbyzincchlorideactivationofsugarcanebagasse[J].JournalofPowerSources,2010,195(3):912-918.[23]GUOHL,GAOQM.Boronandnitrogenco-dopedporouscarbonanditsenhancedpropertiesas415supercapacitor[J].JournalofPowerSources,2009,186(2):551-556.[24]XUJ,WUC,YANP,etal.Porecharacteristicsofcarbide-derivedcarbonsobtainedfromcarbideswithdifferentcarbonvolumefractions[J].MicroporousandMesoporousMaterials,2014,198:74-81.[25]LIZ,XUZ,TANX,etal.Mesoporousnitrogen-richcarbonsderivedfromproteinforultra-highcapacitybatteryanodesandsupercapacitors[J].Energy&EnvironmentalScience,2013,6(3):871-878.420[26]FENGD,LVY,WUZX,etal.Free-standingmesoporouscarbonthinfilmswithhighlyorderedporearchitecturesfornanodevices[J].JournaloftheAmericanChemicalSociety,2011,133(38):15148-15156.[27]PandolfoAG,HollenkampAF.Carbonpropertiesandtheirroleinsupercapacitors[J].Journalofpowersources,2006,157(1):11-27.[28]JINZY,LUAH,XUYY,etal.IonicLiquid‐AssistedSynthesisofMicroporousCarbonNanosheetsfor425UseinHighRateandLongCycleLifeSupercapacitors[J].AdvancedMaterials,2014,26(22):3700-3705.[29]GenoveseM,JIANGJ,LIANK,etal.Highcapacitiveperformanceofexfoliatedbiocharnanosheetsfrombiomasswastecorncob[J].JournalofMaterialsChemistryA,2015,3(6):2903-2913.[30]CHENGP,GAOSY,ZANGPY,etal.Hierarchicallyporouscarbonbyactivationofshiitakemushroomforcapacitiveenergystorage[J].Carbon,2015,93:315-324.430[31]MAGH,YANGQ,SUNKJ,etal.Nitrogen-dopedporouscarbonderivedfrombiomasswasteforhigh-performancesupercapacitor[J].Bioresourcetechnology,2015,197:137-142.[32]CHENGP,LIT,YUH,etal.Biomass-derivedcarbonfiberaerogelasabinder-freeelectrodeforhigh-ratesupercapacitors[J].TheJournalofPhysicalChemistryC,2016,120(4):2079-2086.[33]QIANWJ,SUNFX,XUYH,etal.Humanhair-derivedcarbonflakesforelectrochemical435supercapacitors[J].Energy&EnvironmentalScience,2014,7(1):379-386.[34]JAINA,XUCH,JayaramanS,etal.Mesoporousactivatedcarbonswithenhancedporositybyoptimalhydrothermalpre-treatmentofbiomassforsupercapacitorapplications[J].MicroporousandMesoporousMaterials,2015,218:55-61.[35]TIANWQ,GAOQM,TANYL,etal.Bio-inspiredbeehive-likehierarchicalnanoporouscarbonderived440frombamboo-basedindustrialby-productasahighperformancesupercapacitorelectrodematerial[J].JournalofMaterialsChemistryA,2015,3(10):5656-5664.[36]LIJ,WUQ.Waterbamboo-derivedporouscarbonsaselectrodematerialsforsupercapacitors[J].NewJournalofChemistry,2015,39(5):3859-3864.-14-
中国科技论文在线http://www.paper.edu.cn[37]TalloI,ThombergT,KurigH,etal.Novelmicromesoporouscarbonmaterialssynthesizedfromtantalum445hafniumcarbideandtungstentitaniumcarbide[J].Carbon,2014,67:607-616.[38]ZHAOQL,WANGXY,LIUJ,etal.Designandsynthesisofthree-dimensionalhierarchicalorderedporouscarbonsforsupercapacitors[J].ElectrochimicaActa,2015,154:110-118.[39]WANGY,SHIZQ,HUANGY,etal.Supercapacitordevicesbasedongraphenematerials[J].Thejournalofphysicalchemistry.C,Nanomaterialsandinterfaces,2009,113(30):13103-13107.450[40]KalpanaD,ChoSH,LeeSB,etal.Recycledwastepaper-Anewsourceofrawmaterialforelectricdouble-layercapacitors[J].JournalofPowerSources,2009,190(2):587-591.[41]ZHANGXY,WANGXY,JIANGLL,etal.Effectofaqueouselectrolytesontheelectrochemicalbehaviorsofsupercapacitorsbasedonhierarchicallyporouscarbons[J].JournalofPowerSources,2012,216:290-296.[42]GAOJ,WANGXY,ZHAOQL,etal.Synthesisandsupercapacitiveperformanceofthree-dimensional455cubic-orderedmesoporouscarbons[J].ElectrochimicaActa,2015,163:223-231.[43]SeredychM,JurcakovaDH,LUGQ,etal.Surfacefunctionalgroupsofcarbonsandtheeffectsoftheirchemicalcharacter,densityandaccessibilitytoionsonelectrochemicalperformance[J].Carbon,2008,46(11):1475-1488.460竹笋壳基活性炭材料的制备及其超级电容性能研究鲁群,张友为,王先友,高姣,刘佳,陈曼芳,汪形艳(湘潭大学化学学院,湘潭411105)465摘要:生物质多孔碳材料由于具有天然易得,价格低廉的特点,作为一种最有前途的电极材料广泛应用于超级电容器中。本章选用了资源十分丰富、再生周期很短的生物质废弃物(竹笋壳)作为制备活性炭材料的原材料。这种新型的竹笋壳基活性炭材料是通过两步制备的,在氩气气氛下,先将竹笋壳碳化,然后将碳化产物混合KOH进行高温活化而得到的。并研究了KOH的剂量对产物的物理性质以及电容性能的影响。在最佳条件下制备出的碳材2-1470料比表面积高达3408m,在6MKOH电解质中具有杰出的电容性能(308Fg在1A-1g)以及优异的循环稳定性(97.4%循环10,000次)。关键词:超级电容器;竹笋壳;炭化;活化;活性炭中图分类号:O646-15-'
您可能关注的文档
- 柴达木盆地昆北地区路乐河组下干柴沟组泥岩地层地球化学及古环境意义.pdf
- 氮杂环丁烷衍生的锌配合物合成及催化研究.pdf
- 水稻XYLP7基因在维管系统发育中的功能.pdf
- 浪致混合效应的耦合模式模拟.pdf
- 海绵状石墨烯镍颗粒混合结构可拉伸气敏传感器的制备.pdf
- 潜在蒸散发数据对网格HBV模型降雨径流模拟的影响分析.pdf
- 烯丙基荧光素-丙烯酰胺沉淀共聚荧光分子印迹选择性荧光检测三氟氯氰菊酯残留.pdf
- 珠三角地区有机硝酸的模拟及其对臭氧生成的化学影响分析.pdf
- 石墨烯分散液光限幅特性的温度调控.pdf
- 莱维飞行中的轨线动力学.pdf
- 阿勒泰泥炭重金属元素异常记录的人类活动信息.pdf
- 陕西双王金矿床中自然金属与金属互化物.pdf
- 预磁化对SmBCO超导块材磁悬浮性能的影响.pdf
- 高效液相色谱法测定野生和人工种植库鲁木提草中柚皮苷和木犀草素的含量.pdf
- 高能球磨法对Li3Mg2NbO6陶瓷结构及性能的影响.pdf
- 1,25(OH)2D3对谷氨酸诱导HT-22细胞损伤保护作用研究.pdf
- 30种萱草属植物的倍性鉴定.pdf
- 4种独蒜兰属植物光合特性比较.pdf
相关文档
- 施工规范CECS140-2002给水排水工程埋地管芯缠丝预应力混凝土管和预应力钢筒混凝土管管道结构设计规程
- 施工规范CECS141-2002给水排水工程埋地钢管管道结构设计规程
- 施工规范CECS142-2002给水排水工程埋地铸铁管管道结构设计规程
- 施工规范CECS143-2002给水排水工程埋地预制混凝土圆形管管道结构设计规程
- 施工规范CECS145-2002给水排水工程埋地矩形管管道结构设计规程
- 施工规范CECS190-2005给水排水工程埋地玻璃纤维增强塑料夹砂管管道结构设计规程
- cecs 140:2002 给水排水工程埋地管芯缠丝预应力混凝土管和预应力钢筒混凝土管管道结构设计规程(含条文说明)
- cecs 141:2002 给水排水工程埋地钢管管道结构设计规程 条文说明
- cecs 140:2002 给水排水工程埋地管芯缠丝预应力混凝土管和预应力钢筒混凝土管管道结构设计规程 条文说明
- cecs 142:2002 给水排水工程埋地铸铁管管道结构设计规程 条文说明