- 1.57 MB
- 2022-04-22 13:43:50 发布
- 1、本文档共5页,可阅读全部内容。
- 2、本文档内容版权归属内容提供方,所产生的收益全部归内容提供方所有。如果您对本文有版权争议,可选择认领,认领后既往收益都归您。
- 3、本文档由用户上传,本站不保证质量和数量令人满意,可能有诸多瑕疵,付费之前,请仔细先通过免费阅读内容等途径辨别内容交易风险。如存在严重挂羊头卖狗肉之情形,可联系本站下载客服投诉处理。
- 文档侵权举报电话:19940600175。
'中国科技论文在线http://www.paper.edu.cnTheeffectsofactivationconditionsonthesupercapacitive#performanceofporousactivatedbiocharLUQun,ZHANGYouwei,WANGXianyou,GAOJiao,LIUJia,CHENManfang,**5LIUMin,WANGXingyan(SchoolofChemistry,XiangtanUniversity,Xiangtan411105,China)Abstract:TheporouscarbonsderivedfromnaturallyabundantbambooshootshellfortheapplicationofsupercapacitorarefurtheractivatedbychemicalactivationwithZnCl2,H3PO4andK2CO3.Theinfluencesofactivationconditionontheporestructureandphysicochemicalpropertiesofthe10as-preparedporouscarbonsamplesareinvestigatedbyscanningelectronmicroscopy(SEM),transmissionelectronmicroscope(TEM),nitrogenadsorption-desorptionisotherm,cyclicvoltammogram(CV),galvanostaticcharge-discharge(GCD)test,electrochemicalimpedancespectroscopy(EIS)andcyclelifemeasurement.Theresultsshowthattheporousactivatedcarbonpreparedat1:1weightratioofK2CO3tobambooshootshellexhibitsaspecificsurfaceareaof1800-115m2gwiththeaverageporesizeof3.35nm(AC-K2CO3-900).Inaddition,theAC-K2CO3-900shows-1-1ahighspecificcapacitanceof287Fgatthecurrentdensityof1Ag.Moreover,theAC-K2CO3-900supercapacitorholdshighcapacitanceretentionof95.80%evenafter10000cycles,indicatingitspotentialprospectfortheapplicationofhigh-performancesupercapacitors.Keywords:Bambooshootshell;Porouscarbon;Activationconditions;Activatedcarbon;20Supercapacitor0IntroductionTheincreasingenergyshortageandconcomitantenvironmentalpressureforceustodevelop[1]sustainableandrenewableenergyconversionsandelectricalenergystoragesystems.Among25theenergyconversionandstoragesystems,supercapacitorsorelectrochemicalcapacitorsareoneoftheworthiestchoices.Theyareanewkindofenergystoragesystemthatcancombinetheadvantagesofdielectriccapacitorsandrechargeablebatteriestoachievehighpowerdensity,long[2,3]cyclelife,fastcharge-dischargerateandoperationalsafety.Duetotheirexcellentproperties,supercapacitorshavealreadyacquiredgreatattentionofindustryandacademiainrecentyears.30Thus,theyhavealreadybeenwidelyappliedinmanyfields,suchas,renewableenergypower[4]plants,hybridelectricvehicles,electricvehicles,etc..Basically,supercapacitorscanbeclassifiedintotwodifferentcategoriesaspseudo-capacitorsandelectrochemicaldoublelayercapacitors(EDLCs)dependingonthechargestoragemechanism[5,6].IntheEDLCs,thecapacitancecomesfromtheaccumulationofchargesatthe35electrode/electrolyteinterface,whichrequireselectrodematerialswithhighsurfaceareaandpores[7,8]adaptedtothesizeofelectrolyteions.Whileinthepseudo-capacitor,faradaicredoxreactions[9,10]andtheresultantchargetransfertakeplace,resultinginpseudocapacitance.Manyeffortshavebeendevotedtothedevelopmentofsupercapacitorelectrodematerialsinrecentyears.Sofar,thecarbon-basedmaterialsareoneofthemostwidelyusedelectrodematerialsforsupercapacitors,[11]40especiallyfortheEDLCs.Amongthem,activatedcarbonswithuniquecharacteristics,suchasgoodchemicalandthermalstability,highspecificsurfacearea,easyavailabilityandrelativelylow[12,13]cost,arepromisingelectrodematerialsfortheEDLCs.Thechoiceofrawmaterialandtheassociatedactivationprocess(eitherchemicalorphysicalFoundations:theNationalNaturalScienceFoundationofChina(GrantNos.51072173,51272221,51302239),theSpecializedResearchFundfortheDoctoralProgramofHigherEducation(GrantNos.20134301130001and20134301120007).Briefauthorintroduction:LUQun(1993.11-),female,Mastercandidate,majoringinsupercapacitorCorrespondanceauthor:WANGXianyou(1962.9-),male,Professor,majoringinelectrochemistryandenergymaterials.E-mail:wxianyou@126.com-1-
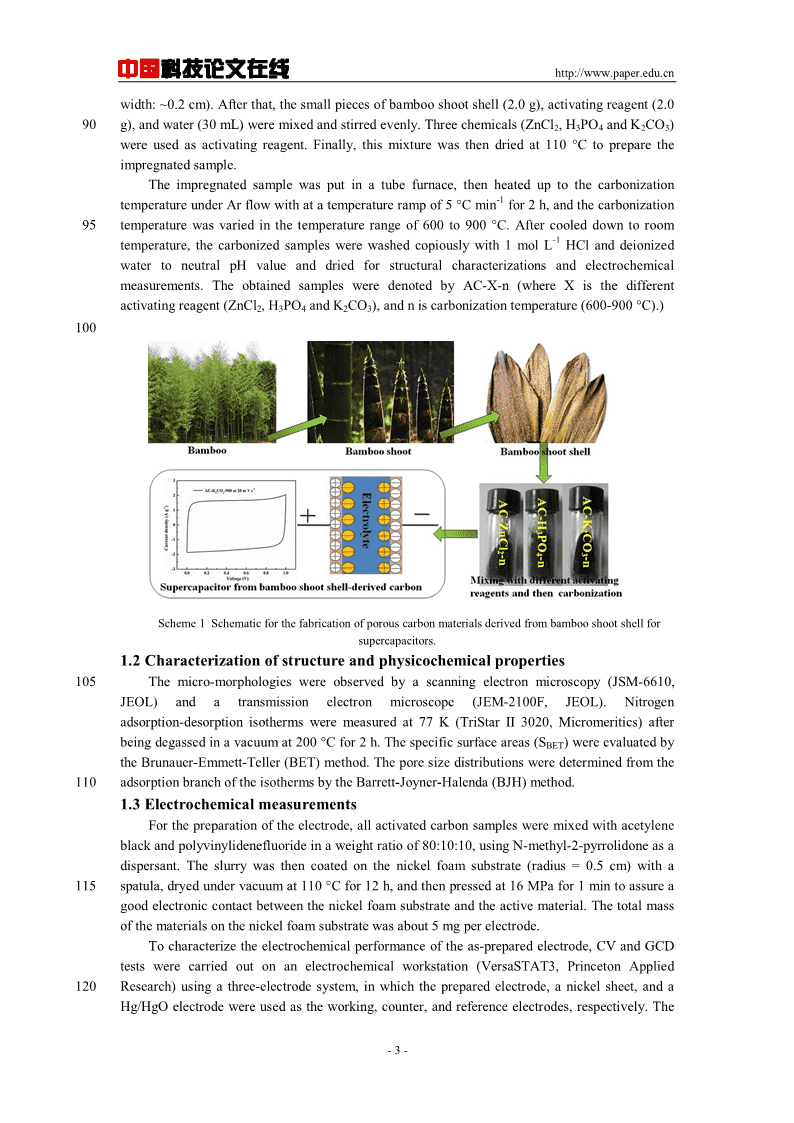
中国科技论文在线http://www.paper.edu.cnactivation)arethemostimportantfactorsthatinfluencetheelectrochemicalperformancesof[14,15]45activatedcarbons.Currently,conventionalactivatedcarbonsaremainlyproducedfromcoal,petroleumcoke,needlecoke,andbiomassprecursors,exhibitingdifferentelectrochemical[16-18]performances,whichsuggesttheimportanceoftheprecursor.Althoughtheactivatedcarbonsproducedfromtraditionalprecursorssuchascoalandcokespossessahighspecificsurfaceareaandgoodcapacitanceproperties,thedifficultiesinthesubsequentactivationprocess[16,18,19]50andtheassociatedenvironmentalpollutionhavebecomethemainissue.Aswellknown,therearegenerallytwoactivationtreatmentmethodsforthepreparationoftheactivatedcarbon:physicalandchemicalactivation.Physicalactivationisconsistedoftwosteps:thecarbonizationoftherawmaterialandthesubsequentactivationoftheporouscarbonizedproductbyusingcarbondioxideorsteam;whileforchemicalactivation,boththecarbonizationandtheactivation55stepcanbeproceedsimultaneously.Therefore,fortheactivatedcarbonsderivedfrombiomassprecursors,chemicalactivationisusuallyused.Forinstance,Chengetal.presentedafacileandeffectivetwo-stepactivationmethodcorrespondingtoH3PO4andKOHusedasactivatingagenttoprepareahierarchicallyporouscarbonwithnaturalshiitakemushroomasthestartingmaterials,whichexhibitedaspecific-1[20]60capacitanceof306and149Fginaqueousandorganicelectrolyte,respectively.Maetal.preparednitrogen-dopedporouscarbonemployingpotatowasteresidueasthecarbonsource,ZnCl2astheactivatingagentandmelamineasnitrogendopingagent,whichpossessedaspecific-1[21]capacitanceof255Fginaqueouselectrolyte.Aktasetal.obtainedporousactivatedcarbonderivedfromwasteteaforapplicationassupercapacitorelectrodes,utilizingachemicalactivation65methodinvolvingtreatmentwitheitherK2CO3orH3PO4,whichexhibitedaspecificcapacitance-1-1[22]of203Fgand123Fg,respectively.Chenetal.reportedthepreparationofactivatedcarbonfromcottonstalkbyusingH3PO4chemicalactivationmethod,whichshowedaspecific-1[23]capacitanceof114Fginorganicelectrolyte.Asweknow,Chinaisoneofthelargestbamboo-plantingcountriesintheworld,andthereis70anaverageof600millionhectaresofbamboo.Bamboo,asabundantnaturalbiomassresources,hasalsobeenextensivelyusedtoprepareactivatedcarbonforsupercapacitorsandlithiumionbatteries.However,bambooshootshell,anotherpromisingbiomassprecursorforthepreparationofactivatedcarbon,hasneveracquiredanyattention,nottomentioninanyelectrochemicalapplication.Bambooshootshellisnaturallypeeledoffduringthegrowingprocessofbamboo,and75alargenumberofbambooshootshellsarelefteveryyear.Thus,bambooshootshellisanabundantandrenewableresourceastheprecursorforthepreparationofactivatedcarbon.Inthispaper,theinnovativesynthesisrouteoftheactivatedcarbonderivedfrombambooshootshellbychemicalactivationwithdifferentactivatingagent(ZnCl2,H3PO4andK2CO3)athightemperatureisputforward.Theinfluencesofpreparationconditions(e.g.,carbonizationtemperatureand80chemicalreagent)ontheporestructureandthesupercapacitiveperformanceoftheactivatedcarbonsderivedfrombambooshootshellarestudiedindetail.1Experimentalsection1.1MaterialpreparationAllreagentsareanalyticalgradewithoutfurtherpurification,andthebambooshootshellwas85collectedfromnearbyvillage.Scheme1showstheproceduretosynthesizethebambooshootshellderivedactivatedcarbonmaterials.Atfirst,thebambooshootshellwaswashedwithrunningwateranddistilledwatertoensurethatallimpuritieswereremovedanddriedoffintheovenat105°C.Then,thedriedbambooshootshellwasfragmentedintosmallpieces(length:~1cm;-2-
中国科技论文在线http://www.paper.edu.cnwidth:~0.2cm).Afterthat,thesmallpiecesofbambooshootshell(2.0g),activatingreagent(2.090g),andwater(30mL)weremixedandstirredevenly.Threechemicals(ZnCl2,H3PO4andK2CO3)wereusedasactivatingreagent.Finally,thismixturewasthendriedat110°Ctopreparetheimpregnatedsample.Theimpregnatedsamplewasputinatubefurnace,thenheateduptothecarbonization-1temperatureunderArflowwithatatemperaturerampof5°Cminfor2h,andthecarbonization95temperaturewasvariedinthetemperaturerangeof600to900°C.Aftercooleddowntoroom-1temperature,thecarbonizedsampleswerewashedcopiouslywith1molLHClanddeionizedwatertoneutralpHvalueanddriedforstructuralcharacterizationsandelectrochemicalmeasurements.TheobtainedsamplesweredenotedbyAC-X-n(whereXisthedifferentactivatingreagent(ZnCl2,H3PO4andK2CO3),andniscarbonizationtemperature(600-900°C).)100Scheme1Schematicforthefabricationofporouscarbonmaterialsderivedfrombambooshootshellforsupercapacitors.1.2Characterizationofstructureandphysicochemicalproperties105Themicro-morphologieswereobservedbyascanningelectronmicroscopy(JSM-6610,JEOL)andatransmissionelectronmicroscope(JEM-2100F,JEOL).Nitrogenadsorption-desorptionisothermsweremeasuredat77K(TriStarII3020,Micromeritics)afterbeingdegassedinavacuumat200°Cfor2h.Thespecificsurfaceareas(SBET)wereevaluatedbytheBrunauer-Emmett-Teller(BET)method.Theporesizedistributionsweredeterminedfromthe110adsorptionbranchoftheisothermsbytheBarrett-Joyner-Halenda(BJH)method.1.3ElectrochemicalmeasurementsForthepreparationoftheelectrode,allactivatedcarbonsamplesweremixedwithacetyleneblackandpolyvinylidenefluorideinaweightratioof80:10:10,usingN-methyl-2-pyrrolidoneasadispersant.Theslurrywasthencoatedonthenickelfoamsubstrate(radius=0.5cm)witha115spatula,dryedundervacuumat110°Cfor12h,andthenpressedat16MPafor1mintoassureagoodelectroniccontactbetweenthenickelfoamsubstrateandtheactivematerial.Thetotalmassofthematerialsonthenickelfoamsubstratewasabout5mgperelectrode.Tocharacterizetheelectrochemicalperformanceoftheas-preparedelectrode,CVandGCDtestswerecarriedoutonanelectrochemicalworkstation(VersaSTAT3,PrincetonApplied120Research)usingathree-electrodesystem,inwhichthepreparedelectrode,anickelsheet,andaHg/HgOelectrodewereusedastheworking,counter,andreferenceelectrodes,respectively.The-3-
中国科技论文在线http://www.paper.edu.cnCVmeasurementswereperformedbetween-1and0Vatdifferentscanrates.TheGCDmeasurementswereconductedfrom-1Vto0Vatdifferentcurrentdensities.EISwasusedtoevaluatetheimpedancebehaviorsofthepreparedsupercapacitorbytheelectrochemical125workstationonacoin-typesupercapacitorassembledbytwoelectrodeswithaseparatorbetween5-2them.TheEISwasmeasuredinthefrequencyrangeof10Hzto10Hzwithamplitudeof5mV.Thecyclelifewasmeasuredonthiscoin-typesupercapacitoratthevoltagewindowof0-1Vata-1currentdensityof1Agbyasupercapacitorteststation(SCTS,ArbinInstruments).Besides,all-1thetestswerecarriedoutin6molLKOHelectrolyteatabout25°C.1302.ResultsanddiscussionToinvestigatetheinfluencesofactivationconditionsontheporetextureandspecificsurfaceareaoftheas-preparedactivatedcarbons,thenitrogenadsorption/desorptionisothermswereperformed.Fig.1(a)-(b)presentthetypicalN2adsorption-desorptionisothermsandtheBJHporesizedistributionsoftheactivatedcarbonsbyZnCl2activation.AsshowninFig.1(a),allthe135AC-ZnCl2-n(n=600-900)samplesshowasimilartypeIsorptionisotherm,andAC-ZnCl2-600displaysthehighestsorptioncapacityamongallthesamples.Atlowrelativepressureregion(P/P0<0.05),thesharpincreaseobservedinallsamplesindicatestheadsorptioninabundant[13]micropores.Theslightlyupwardslopesofallsamplesatthemediumrelativepressureregion[24]confirmthepresenceofmesopores.Inaddition,asmallhysteresisloopcanbeseenfor140AC-ZnCl2-800andAC-ZnCl2-900,whichisthecapillarycondensationinthemesoporesderiving[21]fromthecollapseofthemicroporesduringtheZnCl2activation.WiththeincreaseofthecarbonizationtemperatureduringthechemistryactivationprocessofZnCl2,theadsorptioncapacityoftheas-preparedcarbonsampledecreases.TheporesizedistributionsofallthesamplesareillustratedinFig.1(b).Asshown,withtheincreaseofthecarbonizationtemperature,thepore145sizeofthepreparedcarbonsamplesalsoenlargesslightly.ThecorrespondingBETspecificsurfacearea,porevolumesandporesizesofAC-ZnCl2-naresummarizedinTable1.ItcanbefoundfromTable1thatthespecificsurfaceareasoftheAC-ZnCl2-nsamplesdecreasebetween2-1thetemperatureof600and900°C,andthemaximumspecificsurfaceareasof1697mgwith3-1thelargestporevolumeof0.89cmgisobservedat600°C.ThisindicatesthatZnCl2works150moreeffectivelyasactivatingreagentsat600°C.Table1TexturalparametersofAC-ZnCl2-nobtainedfromnitrogenadsorption-desorptionisotherms.sampleBETsurfaceareaPorevolumeMicroporevolumePorediameter2-13-13-1(mg)(cmg)(cmg)(nm)AC-ZnCl2-60016970.890.612.75AC-ZnCl2-70015230.830.532.82AC-ZnCl2-80014460.750.492.86AC-ZnCl2-90014030.690.432.87Fig.1(c)-(d)showthetypicalN2adsorption-desorptionisothermsandtheBJHporesizedistributionsoftheas-preparedactivatedcarbonsbyH3PO4activation.Aspresented,allthecurvesofAC-H3PO4-n(n=600-900)displayasimilartypeIVisothermwithobviouslyhysteresisloop.155Attheregionoflowrelativepressure,thesharpincreasecanbeobserved,whichsuggeststheexistenceofmicropores.Atthesametime,thehysteresisloopsatthemediumrelativepressure[13]suggestamesoporousstructuresinallthesamples.ItcanbeeasilyseenthatAC-H3PO4-700exhibitsthehighestsorptioncapacityamongallthesamples.Theporesizedistributionsofallthe-4-
中国科技论文在线http://www.paper.edu.cnsamplesbyH3PO4activationaredisplayedinFig.1(d).Itcanbefoundthatallthesamplesshowa160similarsharpwithaweakpeakatca.3.5nm,whichcanbeinterpretedasthemesoporesgeneratedbyH3PO4activation.ThecorrespondingBETspecificsurfaceareas,porevolumesandporesizesofAC-H3PO4-naresummarizedinTable2.ItcanbeseenfromTable2thatthecorresponding2-12-1specificsurfaceareasincreasefrom1034mgto1260mgfirstlyandthengraduallydecrease2-1to1174mgwiththetemperaturerangingfrom600°Cto900°C.TheAC-H3PO4-700exhibits165thehighestspecificsurfacearea,suggestingthatH3PO4worksmoreeffectivelyasactivatingreagentsat700°C.Table2TexturalparametersofAC-H3PO4-nobtainedfromnitrogenadsorption-desorptionisotherms.sampleBETsurfaceareaPorevolumeMicroporevolumePorediameter2-13-13-1(mg)(cmg)(cmg)(nm)AC-H3PO4-60010340.690.343.01AC-H3PO4-70012600.690.462.89AC-H3PO4-80012140.650.432.97AC-H3PO4-90011940.640.423.03Fig.1(e)-(f)displaythetypicalN2adsorption-desorptionisothermsandtheBJHporesizedistributionsoftheas-preparedactivatedcarbonsbyK2CO3activation.AspresentedinFig.1(e),170alltheAC-K2CO3-n(n=600-900)samplesshowasimilartypeIsorptionisotherm,andAC-K2CO3-900displaysthehighestsorptioncapacityamongallthesamples.Theearlystageoftheisothermswiththesharpincreasecorrespondstomicroporefilling;thesloppyregionatahigh[24]relativepressureindicatesthemultilayeradsorptiononthemesopores.Inaddition,withtheincreaseofthecarbonizationtemperature,theadsorptioncapacityoftheas-preparedcarbon175samplesalsoincrease,andthehysteresisloopscanbeobservedmoreeasily.Besides,theporesizedistributionsofallthesamplesareillustratedinFig1(f).Asshown,withtheincreaseofthecarbonizationtemperature,theporesizeofthepreparedcarbonsamplesalsoenlarges.ThecorrespondingBETspecificsurfaceareas,porevolumesandporesizesofAC-K2CO3-naresummarizedinTable3.AsbeingseenfromTable3,whenthecarbonizationtemperatureisvaried180from600°Cto900°C,thecorrespondingspecificsurfaceareasofAC-K2CO3-nincreasefrom2-12-1722mgto1800mg.Obviously,theAC-K2CO3-900givesthehighestspecificsurfaceareasamongalltheK2CO3activationsamples,indicatingthatK2CO3worksmoreeffectivelyasactivatingreagentsat900°C.Table3TexturalparametersofAC-K2CO3-nobtainedfromnitrogenadsorption-desorptionisotherms.sampleBETsurfaceareaPorevolumeMicroporevolumePorediameter2-13-13-1(mg)(cmg)(cmg)(nm)AC-K2CO3-6007220.420.272.58AC-K2CO3-7009580.520.382.66AC-K2CO3-80013650.710.552.86AC-K2CO3-90018001.000.653.35185-5-
中国科技论文在线http://www.paper.edu.cnFig.1(a)nitrogenadsorption-desorptionisothermsand(b)poresizedistributioncurvesofAC-ZnCl2-npreparedatdifferentcarbonizationtemperature;(c)Nitrogenadsorption-desorptionisothermsand(d)poresizedistributioncurvesofAC-H3PO4-npreparedatdifferentcarbonizationtemperature;(e)Nitrogenadsorption-desorption190isothermsand(f)poresizedistributioncurvesofAC-K2CO3-npreparedatdifferentcarbonizationtemperature.Basedontheaboveresults,wechooseAC-ZnCl2-600,AC-H3PO4-700andAC-K2CO3-900asrepresentativesamplestocomparethemorphologyandmicrostuctureofthepreparedcarbonsamples,andtheirSEMandTEMimagesareshownatFig.2.ItcanbeobviouslyfoundinFig.2(a)thattheAC-ZnCl2-600showsabulkstructurewithrelativelysmoothsurface,andthe195obtainedcarbonparticleshaveasizeofabout5μm.Besides,itcanbeseenfromtheTEMimagesinFig.2(b)-(c)thattheAC-ZnCl2-600ownsawell-definedporousstructure;whileinFig.2(d)-(f)thestructureofAC-H3PO4-700issimilartoAC-ZnCl2-600,whichalsoexhibitsabundantporestructure.Incontrast,themorphologyandmicrostructureofAC-K2CO3-900(Fig.2(g)-(i))showcompletelydifferentresults,itexhibitsaquasi-honeycombstructureinwhichtheobviousmicro-200ormeso-poresexistonthewallofmacropores.Suchamorphologyandmicrostructuremaybebeneficialtofacileelectrolyteiontransportandprovidemuchmoreactivesitesforthedouble-layerformationacrosstheinterface,andthusresultinginmorefavorablesupercapacitivebehavior.-6-
中国科技论文在线http://www.paper.edu.cn205Fig.2SEMimages(a,d,g)andTEMimages(b-c,e-f,h-i)ofAC-ZnCl2-600(a-c),AC-H3PO4-700(d-f),andAC-K2CO3-900(g-i).Toinvestigatetheelectrochemicalcharacteristicsofthepreparedcarbonsamples(AC-ZnCl2-600,AC-H3PO4-700,andAC-K2CO3-900),thecyclicvoltammograms(CV)are-1performedwithinapotentialrangeof-1Vto0Vatthescanrateof5mVs.AsshowninFig.2103(a),theCVcurvesforallthesamplesaregenerallyquasi-rectangularshape,suggestinggoodreversibilityandsupercapacitivebehaviors.Moreover,thespecificcapacitanceoftheprepared[25]carbonsamplescanbecalculatedbyEq.(1):1|VfinalI|Cd=V(1)S2ΔmVVinitial(/)dVdtwhereCsisthespecificcapacitance,misthemassofactivematerial,ΔVisthepotential215range,Vinitial/finalisthestarting/terminatepotentialinonecycle,|I|istheresponsecurrent,anddV/dtisthescanrate.ThespecificcapacitanceofAC-ZnCl2-600,AC-H3PO4-700,and-1-1-1-1AC-K2CO3-900are226Fg,184Fgand249Fgatascanrateof5mVs,respectively.Notably,theAC-K2CO3-900ownsthehighestspecificcapacitanceamongallthreesamples.Inaddition,Fig.3(b)showstheCVcurvesoftheAC-K2CO3-900atdifferentscanrates.It220canbeseenthatalltheCVcurvesexhibitaquasi-rectangularshapewithoutobviousdistortion-1-1withthescanrateincreasingfrom5mVsto50mVs,suggestinggoodreversibilityandsupercapacitivebehaviors.TheoutstandingelectrochemicalperformanceoftheAC-K2CO3-900maybeattributedtoitshighspecificsurfaceareaandlargerporevolume.Tofurtherestimatetheelectrochemicalperformanceofthesamples,theGCDtestsfor225AC-ZnCl2-600,AC-H3PO4-700andAC-K2CO3-900samplesareperformedwithinapotential-1rangeof-1Vto0Vatacurrentdensityof1Ag.AsshowninFig.3(c),theGCDcurvesofallthesamplesshowagenerallysymmetricalshapeandlinearrelationshipwithtime,suggestingthetypicalcapacitivebehaviour.Especially,theAC-K2CO3-900possessesthemaximumchargingand-7-
中国科技论文在线http://www.paper.edu.cndischargingtimes,whichtestifiesthatAC-K2CO3-900exhibitsthehighestspecificcapacitance230amongallsamples.Moreover,thespecificcapacitancesoftheelectrodematerialscanbe[21]calculatedfromtheGCDcurveaccordingtoEq.(2):CItmV=(×Δ)/(×Δ)(2)m-1whereCm,I,Δt,ΔV,andmarethespecificcapacitance(Fg),thedischargecurrent(A),thedischargetime(s),thepotentialwindow(V),andtheweightofactivematerial(g)intheworking235electrode,respectively.ThespecificcapacitanceofAC-ZnCl2-600,AC-H3PO4-700,and-1-1AC-K2CO3-900atacurrentdensityof1Agare256,200,and287Fg,respectively.Obviously,thespecificcapacitanceofAC-K2CO3-900ismuchhigherthanthoseofAC-ZnCl2-600andAC-H3PO4-700.Itcanbeascribedtothelargespecificsurfaceareaandappropriatelyporous[13]structure,consequentlyleadingtohighercapacitancestorage.Furthermore,theGCDcurvesof240AC-K2CO3-900atdifferentcurrentdensitiesarealsopresentedintheFig.3(d).Withtheincreaseofcurrentdensity,theGCDcurvesstillshowagoodsymmetricalshape,suggestingthatitownsexcellentreversibilityandgoodsupercapacitivecharacteristic.Inaddition,thespecificcapacitance-1-1-1ofAC-K2CO3-900reaches287Fgat1Agandstillremains208Fgatahighcurrentdensity-1of5Ag.245-1Fig.3CVcurvesof(a)theAC-ZnCl2-600,AC-H3PO4-700andAC-K2CO3-900electrodesat5mVsand(b)theAC-K2CO3-900electrodeatdifferentscanratesand(c)theGCDcurvesoftheAC-ZnCl2-600,AC-H3PO4-700-1andAC-K2CO3-900electrodesat1Agand(d)theGCDcurvesoftheAC-K2CO3-900atdifferentcurrentdensities.250Inordertocharacterizetheimpedancebehaviorsofthepreparedcarbonmaterials,theelectrochemicalimpedancespectroscopy(EIS)isperformedintheopen-circuitvoltage.Fig.4(a)showstheNyquistplotsofthesupercapacitorsusingAC-ZnCl2-600,AC-H3PO4-700,and5-2AC-K2CO3-900aselectrodematerialsinthefrequencyrangefrom10to10Hz.TheNyquistplotsofallthesupercapacitorscanbedividedintothreeparts,includingansemicircleathigh255frequencyregion,ashortlinewithaslopeabout45°inmiddlefrequencyregionandastraightline-8-
中国科技论文在线http://www.paper.edu.cnatlowfrequencyregion,whichisalmostverticaltotheimaginaryaxis(Fig4a),signifyingthe[26-28]performancetendingtothatofanidealcapacitor,asionmigrationarenothindered.Theequivalentseriesresistance(ESR)valuescanbeobtainedfromx-interceptswiththerealaxisoftheNyquistplotsinhighfrequencypart.Inaddition,thesumofESR,RCEandtheinternal260distributionoftheelectrolyteresistancevaluesintheporematrixofcarbonelectrodes(Rpore),i.e.,thetotalpolarizationresistance,isobtainedbyextrapolatingthelow-frequencylinearpartof-Z""vs.Z"plotstoZ""=0.ItcanbeclearlyseenthatthetotalpolarizationresistancesofAC-ZnCl2-600,AC-H3PO4-700,andAC-K2CO3-900supercapacitorsarelow,especiallytheAC-K2CO3-900supercapacitorexhibitsthelowestresistance.265Furthermore,thecapacitance-frequencyplotsareshowninFig.4(b).ThespecificcapacitancevaluesareestimatedbyCm=−1/(2πƒZ"")(3)-1whereC,f,Z″,andmarethespecificcapacitance(Fg),thefrequency(Hz),theimaginary[29]impedance(Ω)andthetotalmassofactivematerial(g),respectively.Itcanbefoundthatthe-1270specificcapacitanceoftheAC-K2CO3-900electrodeis244Fginthefrequencyregionsof0.01-1-1Hz,whichishigherthanthoseofAC-ZnCl2-600(204Fg)andAC-H3PO4-700(171Fg)electrodes.Generallyspeaking,thehighspecificsurfacearea,largeporevolumeandappropriate[21]poresizecanfacilitateelectrolyteionadsorptionontheinnersurfaceofthepores.Fig.4(c)showsBodeplotsofAC-ZnCl2-600,AC-H3PO4-700andAC-K2CO3-900.Itcanbefoundthattheo275phaseanglesofAC-ZnCl2-600,AC-H3PO4-700andAC-K2CO3-900supercapacitorsare-83.9,oo-84.5and-84.7,respectively.Thesefurtherdemonstratethegoodcapacitivefeatureofbambooshootshell-derivedactivatedcarbons,especiallytheAC-K2CO3-900.Moreover,Fig.4(d)showstheseriescapacitancevalues(Cs)atω→0andtheparallelcapacitance(Cp),andCs,Cpare[30]calculatedaccordingtothefollowingEq.(4)and(5),respectively:280Cj=1/(ω″)Z(4)s2C=Z/(Z″||ω)(5)pwhere|Z|istheimpedancemodulus,andωistheangularfrequency.Allthecurvesshowatypicaldropofcapacitancewiththeincreaseofthefrequency(Fig.4(d)).Usually,fortheideally[31]polarizationsystem,Cp/Cs=1.ThesmalldeviationofCp/Csabout0.011,0.009and0.0088285from1inlowfrequencyareobservedfortheAC-ZnCl2-600,AC-H3PO4-700,andAC-K2CO3-900supercapacitors,respectively,indicatinganearlyidealpolarisability.Therefore,itcanbeconcludedthattheimpedancebehaviorofAC-K2CO3-900issuperiortothoseofAC-ZnCl2-600andAC-H3PO4-700.-9-
中国科技论文在线http://www.paper.edu.cn290Fig.4(a)Nyquistplotswiththeexpandedhigh-frequencyregionoftheplotinset,(b)capacitance-frequencyplots,(c)Bodephaseangleplotsand(d)CP/CSvs.frequencydependenciesoftheAC-ZnCl2-600,AC-H3PO4-700,andAC-K2CO3-900supercapacitors.Longcyclelifeofsupercapacitorisoneofthemostimportantparametersforpracticalapplications.Toestimatethelong-termcyclicstability,theAC-ZnCl2-600,AC-H3PO4-700,and-1295AC-K2CO3-900supercapacitorsaretestedfor10000cyclesatacurrentdensityof1Ag.AspresentedinFig.5,theinitialspecificcapacitancesofAC-ZnCl2-600,AC-H3PO4-700and-1AC-K2CO3-900areupto254,194and286Fg,andthespecificcapacitancestillremain243,-1179,and274Fg(ca.95.67%,92.27%,and95.80%ofcapacitanceretention)after10000cycles,respectively.ItcanbeeasilyfoundthatthecycleperformanceofAC-K2CO3-900issuperiorto300thoseofAC-ZnCl2-600andAC-H3PO4-700.Thus,AC-K2CO3-900canbeusedasapromisingelectrodematerialfortheapplicationofhigh-performancesupercapacitors.Fig.5CyclicperformanceofAC-ZnCl2-600,AC-H3PO4-700,andAC-K2CO3-900atacurrentdensityof1-1Ag.3053.Conclusions-10-
中国科技论文在线http://www.paper.edu.cnTheporousactivatedcarbonswithexcellentsupercapacitivebehaviorhavebeensuccessfullypreparedfromnaturallyabundantbambooshootshellbychemicalactivationwithZnCl2,H3PO4andK2CO3attemperaturerangefrom600-900°C.TheAC-K2CO3-900synthesizedat1:1weight-1-1ratioofK2CO3tobambooshootshellshowsahighspecificcapacitanceof286Fgin6molL-1310KOHelectrolyteatthecurrentdensityof1Ag.Furthermore,thespecificcapacitancekeepsstill-1at274Fgwiththeretentionratioof95.80%evenafter10000cyclesatthecurrentdensityof1-1Ag.TheexcellentelectrochemicalperformancesofAC-K2CO3-900maybeattributedtoahigh2-13-1specificsurfacearea(1800mg)andalargeporevolume(1.00cmg),whichprovidealargenumberofreactionsitestoresultintheaccumulationofchargecarriersandhighspecific315capacitance.Therefore,theAC-K2CO3-900willbeaverypromisingcandidateastheelectrodematerialsofhighperformancesupercapacitors.AcknowledgementsThisworkwasfinanciallysupportedbytheNationalNaturalScienceFoundationofChina320(GrantNos.51072173,51272221,51302239),theSpecializedResearchFundfortheDoctoralProgramofHigherEducation(GrantNos.20134301130001and20134301120007).References[1]CHENLF,HUANGZH,LIANGHW,etal.Flexibleall-solid-statehigh-powersupercapacitorfabricated325withnitrogen-dopedcarbonnanofiberelectrodematerialderivedfrombacterialcellulose[J].Energy&EnvironmentalScience,2013,6(11):3331-3338.[2]WANGK,MENGQH,ZHANGYJ,etal.High‐performancetwo‐plyyarnsupercapacitorsbasedoncarbonnanotubesandpolyanilinenanowirearrays[J].Advancedmaterials,2013,25(10):1494-1498.[3]SimonP,GogotsiY.Materialsforelectrochemicalcapacitors[J].Naturematerials,2008,7(11):845-854.330[4]HAOL,LIX,ZHIL.Carbonaceouselectrodematerialsforsupercapacitors[J].AdvancedMaterials,2013,25(28):3899-3904.[5]GUW,SevillaM,MagasinskiA,etal.Sulfur-containingactivatedcarbonswithgreatlyreducedcontentofbottleneckporesfordouble-layercapacitors:acasestudyforpseudocapacitancedetection[J].Energy&EnvironmentalScience,2013,6(8):2465-2476.335[6]YUNYS,CHOSY,SHIMJY,etal.Microporouscarbonnanoplatesfromregeneratedsilkproteinsforsupercapacitors[J].AdvancedMaterials,2013,25(14):1993-1998.[7]KimK,ChoiM,RyooR.Ethanol-basedsynthesisofhierarchicallyporouscarbonusingnanocrystallinebetazeolitetemplateforhigh-rateelectricaldoublelayercapacitor[J].Carbon,2013,60:175-185.[8]LEIC,AminiN,MarkoulidisF,etal.Activatedcarbonfromphenolicresinwithcontrolledmesoporosityfor340anelectricdouble-layercapacitor(EDLC)[J].JournalofMaterialsChemistryA,2013,1(19):6037-6042.[9]KimSY,JeongHM,KwonJH,etal.Nickeloxideencapsulatednitrogen-richcarbonhollowsphereswithmultiporosityforhigh-performancepseudocapacitorshavingextremelyrobustcyclelife[J].Energy&EnvironmentalScience,2015,8(1):188-194.[10]CHENH,HULF,CHENM,etal.Nickel-CobaltLayeredDoubleHydroxideNanosheetsforHigh‐345performanceSupercapacitorElectrodeMaterials[J].AdvancedFunctionalMaterials,2014,24(7):934-942.[11]SimonP,GogotsiY.Capacitiveenergystorageinnanostructuredcarbon-electrolytesystems[J].Accountsofchemicalresearch,2012,46(5):1094-1103.[12]InagakiM,KonnoH,TanaikeO.Carbonmaterialsforelectrochemicalcapacitors[J].Journalofpowersources,2010,195(24):7880-7903.350[13]KarthikeyanK,AmareshS,LeeSN,etal.ConstructionofHigh‐Energy‐DensitySupercapacitorsfromPine‐Cone‐DerivedHigh‐Surface‐AreaCarbons[J].ChemSusChem,2014,7(5):1435-1442.[14]SUDS,SchlöglR.Nanostructuredcarbonandcarbonnanocompositesforelectrochemicalenergystorageapplications[J].ChemSusChem,2010,3(2):136-168.[15]FrackowiakE,BeguinF.Carbonmaterialsfortheelectrochemicalstorageofenergyincapacitors[J].Carbon,3552001,39(6):937-950.[16]PandolfoAG,HollenkampAF.Carbonpropertiesandtheirroleinsupercapacitors[J].Journalofpowersources,2006,157(1):11-27.[17]RedondoE,GonzálezJC,GoikoleaE,etal.Effectofporetextureonperformanceofactivatedcarbonsupercapacitorelectrodesderivedfromolivepits[J].ElectrochimicaActa,2015,160:178-184.360[18]WEIL,YushinG.Nanostructuredactivatedcarbonsfromnaturalprecursorsforelectricaldoublelayer-11-
中国科技论文在线http://www.paper.edu.cncapacitors[J].NanoEnergy,2012,1(4):552-565.[19]BiswalM,BanerjeeA,DeoM,etal.Fromdeadleavestohighenergydensitysupercapacitors[J].Energy&EnvironmentalScience,2013,6(4):1249-1259.[20]CHENGP,GAOSY,ZANGPY,etal.Hierarchicallyporouscarbonbyactivationofshiitakemushroomfor365capacitiveenergystorage[J].Carbon,2015,93:315-324.[21]MAGF,YANGQ,SUNKJ,etal.Nitrogen-dopedporouscarbonderivedfrombiomasswasteforhigh-performancesupercapacitor[J].Bioresourcetechnology,2015,197:137-142.[22]InalIIG,HolmesSM,BanfordA,etal.Theperformanceofsupercapacitorelectrodesdevelopedfromchemicallyactivatedcarbonproducedfromwastetea[J].AppliedSurfaceScience,2015,357:696-703.370[23]CHENM,KANGXY,WumaierT,etal.Preparationofactivatedcarbonfromcottonstalkanditsapplicationinsupercapacitor[J].JournalofSolidStateElectrochemistry,2013,17(4):1005-1012.[24]ZHAOQL,WANGXY,XIAH,etal.Design,preparationandperformanceofnovelthree-dimensionalhierarchicallyporouscarbonforsupercapacitors[J].ElectrochimicaActa,2015,173:566-574.[25]ZHAOQL,WANGXY,LIUJ,etal.SurfaceModificationandPerformanceEnhancementofCarbon375DerivedfromChromiumCarbideforSupercapacitorApplications[J].JournalofTheElectrochemicalSociety,2015,162(6):A845-A851.[26]QIANWJ,SUNFX,XUYH,etal.Humanhair-derivedcarbonflakesforelectrochemicalsupercapacitors[J].Energy&EnvironmentalScience,2014,7(1):379-386.[27]TalloI,ThombergT,KurigH,etal.Novelmicromesoporouscarbonmaterialssynthesizedfromtantalum380hafniumcarbideandtungstentitaniumcarbide[J].Carbon,2014,67:607-616.[28]ZHAOQL,WANGXY,LIUJ,etal.Designandsynthesisofthree-dimensionalhierarchicalorderedporouscarbonsforsupercapacitors[J].ElectrochimicaActa,2015,154:110-118.[29]KalpanaD,ChoSH,LeeSB,etal.Recycledwastepaper-Anewsourceofrawmaterialforelectricdouble-layercapacitors[J].JournalofPowerSources,2009,190(2):587-591.385[30]ZHANGXY,WANGXY,JIANGLL,etal.Effectofaqueouselectrolytesontheelectrochemicalbehaviorsofsupercapacitorsbasedonhierarchicallyporouscarbons[J].JournalofPowerSources,2012,216:290-296.[31]GAOJ,WANGXY,ZHAOQL,etal.Synthesisandsupercapacitiveperformanceofthree-dimensionalcubic-orderedmesoporouscarbons[J].ElectrochimicaActa,2015,163:223-231.390活化方式对多孔活性生物炭材料的结构及电容性能的影响鲁群,张友为,王先友,高姣,刘佳,陈曼芳,刘敏,汪形艳(湘潭大学化学学院,湘潭411105)395摘要:活性炭材料因其比表面高,价格低廉,导电性好等特点被广泛应用于双电层电容器电极材料。影响活性炭性能的主要两个关键因素是原料和活化方式,本文采用来源丰富的生物质废弃物竹笋壳制备多孔炭材料,而后将其分别浸渍ZnCl2、H3PO4、K2CO3进行活化处理,一步法制备了竹笋壳基活性多孔炭材料。并采用多种手段对材料的物理结构及电容性能进行了表征,探讨了不同活化方式对竹笋壳基活性炭材料的各方面性能的影响。结果表明,笋壳2-1400与K2CO3以质量比为1:1活化制备得到的多孔活性炭比表面积高达1800mg,平均孔径在-1-13.35nm左右。该多孔活性炭在1Ag下比电容高达287Fg。经过10000次循环后,容量保持率为95.80%,表明材料具有良好循环稳定性。该材料在高性能超级电容器中具有良好的应用前景。关键词:竹笋壳;多孔炭;活化条件;活性炭;超级电容器405中图分类号:O646-12-'
您可能关注的文档
- 微囊藻毒素合成酶McyG的N端结构域的结构研究.pdf
- 快充和超长稳定的高度互联Cu-Si合金纳米管锂离子电池阳极材料.pdf
- 提高环境质量的财政基础.pdf
- 星型网络下量子态的制备.pdf
- 棉花XTH基因家族全基因组鉴定及进化分析.pdf
- 欠驱动弹跳机器人着地相运动规划.pdf
- 武汉市腹泻婴幼儿隐孢子虫感染的分子流行病学调查.pdf
- 氧化石墨烯诱导再生丝素蛋白成胶.pdf
- 水通道蛋白的研究进展.pdf
- 温度对镁合金微动磨损行为影响研究.pdf
- 湖南省“四化”协同发展实证研究.pdf
- 漂浮式烘箱风嘴风速的研究.pdf
- 热熔法制备PCL基温敏性药物释放体系及其释药性能研究.pdf
- 热网损耗对热电联合系统风电消纳能力的影响.pdf
- 理论计算钒同位素在溶液中的分馏.pdf
- 癌症相关indels的数据库构建及其特征分析.pdf
- 硫化氢合成酶在SVZ区神经干细胞中的表达鉴定及硫化氢对神经干细胞增殖的影响.pdf
- 神经电生理检测推拿对CCI大鼠感觉功能的影响.pdf
相关文档
- 施工规范CECS140-2002给水排水工程埋地管芯缠丝预应力混凝土管和预应力钢筒混凝土管管道结构设计规程
- 施工规范CECS141-2002给水排水工程埋地钢管管道结构设计规程
- 施工规范CECS142-2002给水排水工程埋地铸铁管管道结构设计规程
- 施工规范CECS143-2002给水排水工程埋地预制混凝土圆形管管道结构设计规程
- 施工规范CECS145-2002给水排水工程埋地矩形管管道结构设计规程
- 施工规范CECS190-2005给水排水工程埋地玻璃纤维增强塑料夹砂管管道结构设计规程
- cecs 140:2002 给水排水工程埋地管芯缠丝预应力混凝土管和预应力钢筒混凝土管管道结构设计规程(含条文说明)
- cecs 141:2002 给水排水工程埋地钢管管道结构设计规程 条文说明
- cecs 140:2002 给水排水工程埋地管芯缠丝预应力混凝土管和预应力钢筒混凝土管管道结构设计规程 条文说明
- cecs 142:2002 给水排水工程埋地铸铁管管道结构设计规程 条文说明