- 937.01 KB
- 2022-04-22 13:45:04 发布
- 1、本文档共5页,可阅读全部内容。
- 2、本文档内容版权归属内容提供方,所产生的收益全部归内容提供方所有。如果您对本文有版权争议,可选择认领,认领后既往收益都归您。
- 3、本文档由用户上传,本站不保证质量和数量令人满意,可能有诸多瑕疵,付费之前,请仔细先通过免费阅读内容等途径辨别内容交易风险。如存在严重挂羊头卖狗肉之情形,可联系本站下载客服投诉处理。
- 文档侵权举报电话:19940600175。
'中国科技论文在线http://www.paper.edu.cnConstructionofRecombinantLentivirusVectorTargetingBovineLXRαmRNAandItsSilencingEffectsonBovine#5MuscleSatelliteCells*LiuYongfeng,ZhaoLu,KuTing,YangXingbin(CollegeofFoodEngineeringandNutritionalScience,ShaanxiNormalUniversity,Xi"an710062)Abstract:TheliverXreceptorα(LXRα)isamemberofthenuclearhormonereceptorsuperfamilywhichcouldregulatethetranscriptionofthegenesinvolvedincholesteroltransportation.Recent10studiesshowedthatLXRαisconsideredasacriticalregulatorincholesterolhomeostasisinmacrophages,whichcouldregulateseveralgenesinvolvedincholesteroltransport,suchastheATP-bindingcassettetrans-porters(ABCs),ABCA1,ABCG1,apolipoproteinE(ApoE)andlipoproteinlipase(LPL).Inourstudy,fourpairsofinhibitionshRNAweredesignedtoexclusivelytargetbovineLXRαmRNA.LentiviralvectorcontainingLXRαshRNAswereconstructedthroughBP15andLPrecombinationsystem,andusedtoobtainedthecorrespondinglentivirusesin293Tcells.ThetitersoftheLenti-14MR0054-01,-2,-3and-04viruseswere3×108TU/ml,2×108TU/ml,2.5×108TU/ml,3.5×108TU/ml,respectively.TheknockdownefficiencyofpLenti-03targetingLXRαreached88%inbovinemusclesatellitecells.Furthermore,themRNAexpressionofRXRαwasupregulatedinbovinemusclesatellitecells,whereasthatofPPARα,PPARγ,ABCA1,LPL,ApoE20weredownregulatedat48hpost-pLenti-03virusesinfection.Therefore,theefficientlentivirusvectorinhibitingbovineLXRαexpressionwasobtainedinthepresentstudy,providingbasisforloss-of-functionofLXRαinbovinemusclesatellitecellsKeywords:bovineLXRαgene;lentivirus;vectorconstruction;genesilencing250IntroductionObesity,hypertension,dysmetabolismarediseasescausedbyunhealthydietcustomwhichinfluencedhuman’slivingquality.Thus,alow-cholesterollifestyleisnecessary.Currently,peoplehavepaidmuchattentiontobalancingtheirdietbylow-intakeofcholesterol.Producinggoodqualitymeatwithlow-fat(beef,mutton,pork,etc.)isavitalwaytoprovidepeoplewithhealthymealscondition.30TheliverXreceptorα(LXRα,NR1H3)andLXRβ(NR1H2)aremembersofthenuclearhormonereceptorsuperfamily.LXRαisabundantlyexpressedintheliver,intestine,adiposetissue,kidneyand[1,2]immunemacrophages,whereasLXRβisubiquitouslyexpressed.LiverXreceptors(LXRs)areactivatedinresponsetointracellularlipidaccumulation,whichcouldregulatetranscriptionofanarray[3,ofgenesinvolvedintheregulationofcholesterolhomeostasisandreversecholesteroltransport4]35.CholesterolhomeostasisisintricatelyregulatedbyabatteryoftranscriptionfactorsamongwhichLXRsarenuclearreceptorsthatplayacrucialroleintranscriptionalregulationoflipidmetabolismand[5]inflammation.ActivatedLXRsformaheterodimerwiththeretinoidXreceptorα(RXRα),which[6]bindstoLXRresponsiveelements(LXREs)andconsequentlypromotestargetgeneexpression.LXR/RXRheterodimersarecharacterizedbytheabilitytobeactivatedbyligandinanindependent40manner.Thus,LXR/RXRheterodimersareactivatedbytheRXRligand,e.g.,9-cisretinoicacid,andtheLXRligands,e.g.oxysterols,orareactivatedsynergisticallyinthepresenceofligandsforboth[7]receptors.RecentstudiesshowedthatLXRαisconsideredasacriticalregulatorincholesterolhomeostasisinmacrophages,whichcouldregulateseveralgenesinvolvedincholesteroltransport,suchFoundations:theresearchfundforthedoctoralofhighereducationofChina(20130202120008),theChinapostdoctoralsciencefoundation(2015M570811)andthefundamentalresearchfundsforthecentraluniversitiesofChina(GK201502008).Briefauthorintroduction:LiuYongfeng(1981-),Male,AssociateProfessor,FoodMolecularNutrition.E-mail:yongfeng200@126.com-1-
中国科技论文在线http://www.paper.edu.cnastheATP-bindingcassettetrans-porters(ABCs),ABCA1,ABCG1,apolipoproteinE(ApoE)and[8]45lipoproteinlipase(LPL).[9]ABCA1,ABCG1andApoEarealltargetgenesregulatedbyLXR.Besides,peroxisomeproliferator-activatedreceptor-alpha(PPARα)canactivatethecytochromep450enzymes,resultinginsomeofthehydroxylcholesterolasLXRαendogenousligandfurtheractivateLXRα,whichfurtheractivatingABCA1regulatedcholesterolefflux.RecentstudiesindicatedthatPPARγenhances[10]50cholesteroleffluxbyinducingthetranscriptionofLXRα.PPARγalsoinducestheexpressionofABCA1andABCG1,andpromotescholesteroleffluxfrommacrophagesthroughatranscriptional[11-13]cascademediatedbyLXRα.LXRαcanalsoactivateLPL.Somestudieshaverevealedthatcholesterol-inducedLPLgeneexpressionintheliverisdirectlyregulatedbyRXR/LXRheterodimersin[14]atissue-specificmanner,whichismediatedpredominantlybyLXRαinvivo.55LXRαisimportantforcholesterolmetabolisminhumanandmice.BovineLXRαgenealsocontrolsthecholesterollevelandtheoutflowofcholesteroleffluxinmuscle.GiventhattheimportanceofLXRαgene,regulatingcholesterolinmetabolism,exploringLXRαgenefunctionincholesterolmetabolisminmusclecellstobalancethecholesterolcontentandimprovingthequalityofbeefareofgreatsignificance.PackagingandproliferatinglentiviruswithRNAinterference(RNAi)techniqueisa[15]60conservedbiologicalresponsetodouble-strandedRNA,RNAiisasequence-specificprocessthatregulatesgeneticfunctionsandprovidesdefenseagainstvirusatthepost-transcriptionallevelin[16,17]mammaliancellsandanimals.LentivirusvectorisakindofinactivatedHIVviruswithmanyadvantages,suchashightransfectionrate,stableexpressionintargetcellsandgoodsecurity,soithasbeenwildlyusedintransgenicresearchasacrucialtool.65Lentiviralexpressionvectorisakindoflong-actingsystemwhichcaninfecthostcellswithhigh-efficiency.Thus,weusethismediationsystemtoachievefunctionalgeneresearchonbovinemusclesatelliteprimarycells.ConsideringtheimportanceofLXRαgeneandrelatedstudyonbovinehasnotbeenreportedyet.WehypothesizedthatLXRαmightinfluencesomecholesterolmetabolismregulatinggenes.Toaddressthishypothesis,wesuccessfullyconstructvirusvectorinordertotransfect70bovinecellsanddetectsomecholesterolregulationgenes.Toourknowledge,thisstudyfirstdemonstratedtheinterferenceofLXRαonbovinemusclesatellitecellsinvitrotoinvestigateLXRαgene’sfunctionanditstargetgenes.Furthermore,itcanprovideuswithvaluableinformationforfurtherstudyingofbovineLXRαgenemechanism.1Materialsandmethods75Unlessstatedotherwise,allchemicalsandbiochemicalsusedinthisstudyaretestedforcellcultureandareofmolecularbiologygrade.1.1CellcultureHEK293TCells,thebovinemusclesatellitecellsweregivenbyNBCIC(NationalBeefCattleImprovementCenter,NorthwestA&FUniversity,Yangling,China),andidentifiedwithcellmarkersby80Immunofluoresencestaining,culturedinDulbecco"smodifiedEagle"smedium(DMEM,gibco)withstableL-glutaminesupplementedwith20%fetalbovineserum(FBS,gibco),10%horseserum(HS,gibco),100U/mlpenicillin(sigma),100µg/mlstreptomycin(sigma)(completegrowthmedium),-2-
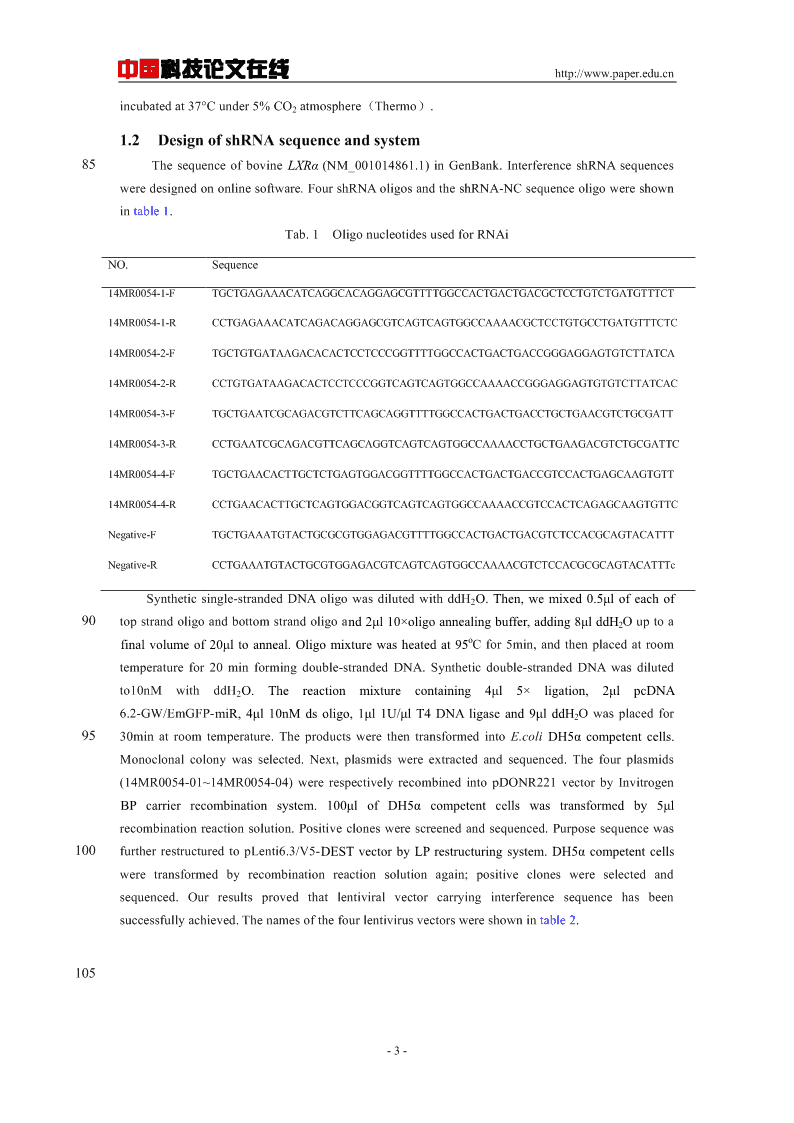
中国科技论文在线http://www.paper.edu.cnincubatedat37°Cunder5%CO2atmosphere(Thermo).1.2DesignofshRNAsequenceandsystem85ThesequenceofbovineLXRα(NM_001014861.1)inGenBank.InterferenceshRNAsequencesweredesignedononlinesoftware.FourshRNAoligosandtheshRNA-NCsequenceoligowereshownintable1.Tab.1OligonucleotidesusedforRNAiNO.Sequence14MR0054-1-FTGCTGAGAAACATCAGGCACAGGAGCGTTTTGGCCACTGACTGACGCTCCTGTCTGATGTTTCT14MR0054-1-RCCTGAGAAACATCAGACAGGAGCGTCAGTCAGTGGCCAAAACGCTCCTGTGCCTGATGTTTCTC14MR0054-2-FTGCTGTGATAAGACACACTCCTCCCGGTTTTGGCCACTGACTGACCGGGAGGAGTGTCTTATCA14MR0054-2-RCCTGTGATAAGACACTCCTCCCGGTCAGTCAGTGGCCAAAACCGGGAGGAGTGTGTCTTATCAC14MR0054-3-FTGCTGAATCGCAGACGTCTTCAGCAGGTTTTGGCCACTGACTGACCTGCTGAACGTCTGCGATT14MR0054-3-RCCTGAATCGCAGACGTTCAGCAGGTCAGTCAGTGGCCAAAACCTGCTGAAGACGTCTGCGATTC14MR0054-4-FTGCTGAACACTTGCTCTGAGTGGACGGTTTTGGCCACTGACTGACCGTCCACTGAGCAAGTGTT14MR0054-4-RCCTGAACACTTGCTCAGTGGACGGTCAGTCAGTGGCCAAAACCGTCCACTCAGAGCAAGTGTTCNegative-FTGCTGAAATGTACTGCGCGTGGAGACGTTTTGGCCACTGACTGACGTCTCCACGCAGTACATTTNegative-RCCTGAAATGTACTGCGTGGAGACGTCAGTCAGTGGCCAAAACGTCTCCACGCGCAGTACATTTcSyntheticsingle-strandedDNAoligowasdilutedwithddH2O.Then,wemixed0.5μlofeachof90topstrandoligoandbottomstrandoligoand2μl10×oligoannealingbuffer,adding8μlddH2Ouptoaofinalvolumeof20μltoanneal.Oligomixturewasheatedat95Cfor5min,andthenplacedatroomtemperaturefor20minformingdouble-strandedDNA.Syntheticdouble-strandedDNAwasdilutedto10nMwithddH2O.Thereactionmixturecontaining4μl5×ligation,2μlpcDNA6.2-GW/EmGFP-miR,4μl10nMdsoligo,1μl1U/μlT4DNAligaseand9μlddH2Owasplacedfor9530minatroomtemperature.TheproductswerethentransformedintoE.coliDH5αcompetentcells.Monoclonalcolonywasselected.Next,plasmidswereextractedandsequenced.Thefourplasmids(14MR0054-01~14MR0054-04)wererespectivelyrecombinedintopDONR221vectorbyInvitrogenBPcarrierrecombinationsystem.100μlofDH5αcompetentcellswastransformedby5μlrecombinationreactionsolution.Positivecloneswerescreenedandsequenced.Purposesequencewas100furtherrestructuredtopLenti6.3/V5-DESTvectorbyLPrestructuringsystem.DH5αcompetentcellsweretransformedbyrecombinationreactionsolutionagain;positivecloneswereselectedandsequenced.Ourresultsprovedthatlentiviralvectorcarryinginterferencesequencehasbeensuccessfullyachieved.Thenamesofthefourlentivirusvectorswereshownintable2.105-3-
中国科技论文在线http://www.paper.edu.cnTab.2ThelentivirusvectorsNO.ThenameoftheplasmidThenameofLentivirusvector114MR0054-01LR-2pLenti-01214MR0054-02LR-2pLenti-02314MR0054-03LR-2pLenti-03414MR0054-04LR-2pLenti-041.3Packaginglentivirus6Whenthe293Tcellsreached70%-80%confluence,cellswereplatedat1.0×10cellsper10cm110cellculturedishandculturedovernight.Themediacanberemoveddirectlybeforetransfection.9μgofPackagingMixand3μgofthelentiviralexpressionplasmidswereaddedto1.5mlOpti-MEMmediumothatwasthenpreheatedat37C.36μllipofectamine2000wasaddedto1.5mlOpti-MEMmediumandmixedgently;then,themixwaskeptatroomtemperaturefor5min.Next,wemixedplasmidsolutionandlipofectamine2000diluentandputthematroomtemperaturefor20min.Then,3mlofPlasmid115liposomecomplexwasaddedintocelldishandincubatedat37°Cin5%CO2for6h,afterwhichweremovedtheprimarymediumandreplaceditwithfreshDMEMwith10%FBS.Thesupernatantwascollectedat48hbycentrifugationat3000rpm/minfor10minandfiltratedby0.45μmmembranefilterunit.Thevirusstocksolutionwasultracentrifugedat50000gfor2h;thesupernatantwasremovedandresuspendedinopti-MEMculturemediumtodeterminethetiter.Lentiviralstocksshouldbealiquoted120andstoredat-80°C.1.4DeterminationofviraltitersTab.3Virusdilutions.StepVirusin100μl-31No.1diluent:5μlviralstock+245μLDFPmedium2×10ml-42No.2diluent:5μlNo.1diluent+245μLDFPmedium2×10ml-53No.3diluent:5μlNo.2diluent+245μLDFPmedium2×10ml-64No.4diluent:5μlNo.3diluent+245μLDFPmedium2×10ml-75No.5diluent:5μlNo.4diluent+245μLDFPmedium2×10ml-87No.6diluent:5μlNo.5diluent+245μLDFPmedium2×10mlNote:DFPmediumisDMEMcontaining20%FBSand8μg/mlPolybrene.3HEK293Tcellswereseededinto96-wellplateatadensityof8×10cellsperwell.Lentiviruswas125dilutedwithDMEMsupplementedwith2%FBSand8μg/mlPolybrene(DFPmedium).Thedetailsoftheviralstockdilutionwereshownintable3.Then,wecarefullyremovedtheculturemediumfrom96-wellplateandreplaceditwith100μldilutedvirussolution.Thecellswereincubatedinat37°Cwith-4-
中国科技论文在线http://www.paper.edu.cn5%CO2.Aftertransfectionfor24hours,GFPexpressionwasobservedunderfluorescencemicroscope,virustiterwascalculatedaftertransfectionfor96hours.LentivirusvectorsofpLenti-01~pLenti-04130weretransducedintoHEK293Tcells,whichwerescreenedunderthesameviewinfluorescentandbrightfieldsofmicroscope.1.5DeterminationofMOIvalueThebovinemusclesatellitecellsweremaintainedinDMEMsupplementedwith20%ofFBSand410%HS.Aftertheconfluence,thecellsweretrypsinizedandcounted,1×10cellswereseededin13596-wellplateswith2mlofmedium,whichwereincubatedovernight.AccordingtoMOIvalueof2,5,10,20,50,100,200,300,wedilutedlenti6.3-RNAiwithDMEMsupplementedwith2%FBSand8μg/mlpolybrene.CellswerelightlywashedoncewithPhosphateBufferedSaline(PBS)andlentivirusliquidwithdifferentMOIvalueswasaddedtothecorrespondingwellsrespectively.After72hours,theexpressionofGFPwasobserved.1401.6RNAextractionTotalRNAwasextractedfrombovinemusclesatellitecellsamplesusingTrizolbuffer,andthefollowingreversewasperformedusingaHigh-capacitycDNAReverseTranscriptionKit.1.7qRT-PCRTheqPCRreactionsystemwas17.3μlultrapurewater,2.5μl10×PCRbuffer,2μlmagnesiumions,1450.2μldNTPs,0.5μlprimers,0.5μl50×sybr,1.2μlcDNAand0.3μlofTaqDNApolymerase.ThePCRamplificationconditionswere:2minat95°C;40cyclesof95°Cfor10s,30sat60°Cand45sat70°C.TheexpressionlevelofmRNAwasdeterminedbycyclethreshold(Ct)normalizedwiththatof−ΔΔCtPPP1R11usingthe2formula.Samplestransfectedwithnegativeinterferencevectorwereusedasacontrol.1501.8DataAnalysisSYBRGreenandcDNAwereusedforQuantitativeReal-timePCR(RT-PCR).Theexpression−ΔΔCtlevelwasquantifiedvia2.AnalyticaldatawasnormalizedtothemRNAexpressionlevelofendogenouscontrolβ-actin.Primersequencesweredescribedintable4.Tab.4PrimerinformationforqRT-PCRGenePrimersequence(5"to3")Productlength(bp)PPARαF:5γ"-TCCCTCTTTGTGGCTGCTAT-3"168bpR:5"-TCGTCAGGATGGTTGTTCTG-3"PPARγF:5"-GCGACTTAGCAATATTTATAGCTGTC-3"105bpR:5"-AGGCTTGCAGCAGATTGTCT-3"RXRαF:5"-GCCTCAATGGTGTCCTCAAAG-3"120bpR:5"-AGCTGTACACCCCGTAGTGCTT-3"ApoEF:5"-AGCTGCTCAACACCCAGGTCATT-3"80bp-5-
中国科技论文在线http://www.paper.edu.cnR:5"-CTCCTTGTAGGCCTTCACCTCCTT-3"ABCA1F:5"-GGATATCCTGAAGCCAGTCCT-3"84bpR:5"-AAAGCTTTTGTGGCTTCACC-3"LPLF:5"-GACAGGATGTGGCCAAGTTT-3"61bpR:5"-TTGCCCAGGGGATAGTTAAA-3"o155Note:Annealingtemperatureforallprimersinthistableis60C.PPARα,peroxisomeproliferator-activatedreceptorα;PPARγ,peroxisomeproliferator-activatedreceptorγ;RXRα,retinoidXreceptorα;ApoE,apolipoproteinE;ABCA1,ATP-bindingcassettetransporterA1;LPL,#Lipoproteinlipase.Theprimersequencesarefrombovine.2Results1602.1Lentiviralpackaging,thetiterdeterminationresults293Tcellswerefirsttransfectedwithlentivirusvectorsfor24hrs,andthenobservedunderfluorescent(Fig.1A-D)andlight(Fig.1a-d)microscopes.SignificantGFPcouldbefoundafter-8transfectionwithvirusin100μLat2×10ml.ThevirustitersforLenti-14MR0054-01,-02,-03and-048888were3×10,2×10,2.5×10and3.5×10TU/ml,respectively.165Fig.1293TcellsweretransfectedwithplasmidscontainingspecificshRNAstargetingLXRαNote:293TcellsweremonitoredaftertransfectionwithpLenti-01,-02,-03and-04lentivirusvectorsat24hunderafluorescencemicroscope(A-D)andalightmicroscope(a-d),respectively.2.2LXRαmRNAexpression170BovinemusclesatellitecellswereinfectedwithpLenti-01,-02,-03and-04lentivirusesfor48h,andthenthesecellswerecollectedqRT-PCRanalysis.WefoundthattheLXRαmRNAexpressionlevelsweredecreasedto0.46,0.23,0.12and0.29ascomparedtonegativecontrollentivirus.Thesilencingefficienciesofthefourviruseswere54%,77%,88%and71%,respectively(Fig.2).-6-
中国科技论文在线http://www.paper.edu.cn175Fig.2KnockdownefficienciesofLXRαshRNAvectorsinbovinemusclesatellitecellsNote:BovinemusclesatellitecellswereinfectedwithpLenti-01,-02,-03and-04lentivirusfor48h,andthentheLXRαmRNAexpressionlevelsweredeterminedbyqRT-PCR.2.3LXRαrelatedgenesmRNAexpressionToexploretheeffectsofLXRαknockdownonLXRαrelatedgenes,theexpressionofPPARα,180PPARγ,ABCA1,LPL,RXRαandapoEwerealsodeterminedwithbyqRT-PCRanalysis,andtheresultsweresummarizedinfig.3.Ascomparedwithnegativecontrolgroup,themRNAexpressionlevelsofPPARα,PPARγ,ABCA1,LPLandapoEweredecreasedinbovinemusclesatellitecellswhenLXRαwasinhibited,whereasthatofRXRαwasincreased(Fig.3).LXRαplayedanegativeroleinregulating.Theresultsshowedthatup-regulationofRXRαexpressionwasinvolvedinLXRαsuppression;185therefore,theexpressionofLXRαandRXRαwerenegativelycorrelated.Fig.3EffectsofLXRαsilencingonmRNAexpressionofrelatedgenesinbovinemusclesatellitecellsNote:Thesedatarepresentthemeans±S.D.*representsP<0.05.TheSameasfollows.ovinemusclesatellitecellswereinfectedwithpLenti-01,-02,-03and-04lentivirusfor48h,andthenthemRNAexpressionlevelsof190(A)PPARα,(B)PPARγ,(C)ABCA1,(D)LPL,(E)ApoEand(F)RXRαweredeterminedbyqRT-PCR.-7-
中国科技论文在线http://www.paper.edu.cnTheinterferenceefficiencyofpLenti-03vectorwas88%,whichwasthebestamongallthevectors.TherelativeexpressionofthesixgenesregulatedbypLenti-03wasshowninfig.4.Comparedwiththenegativecontrolgroup,exceptRXRα,theexpressionleveloftheotherfivegenesdecreasedlessthanone.TherelatedgenesdecreasedbyLXRαwereintheorderofPPARγ>PPARα>LPL>195apoE>ABCA1.TherelativeexpressionofRXRαgenewashigherthanthenegativecontrolandwasup-regulated.Hence,thesilencingeffectsofbovineLXRαgeneonPPARα,PPARγ,ABCA1,LPL,RXRαandapoEweresignificant.Fig.4TheeffectsofpLenti-03infectiononbovinemusclesatellitecells200Note:RelativemRNAexpressionofPPARα,PPARγ,ABCA1,LPL,ApoEandRXRαgenes.3DiscussionBovinemusclesatellitecellswereusedinourexperiment.thepositiverateofGFPwasveryhighwhencellwasinfectedbyadenoviruswithMOIvalue3000,butthepositiveofcellularfluorescenceintensitywasverylow.Thereasonforthiswasthatadenoviruscannotbeappliedtoinfectbovine205musclesatellitecells.However,thepositiverateofthecellswasveryhighwhenlentivirusinfectingbovinemusclesatellitecellswithMOI300.Althoughthepositiveratewaslowandcellularfluorescenceintensitywasweak,whichalsohaveanobviouseffectonthecellularshape.Lentiviralvectorwasdevelopedbasisonthegenetherapyofthehumanimmunodeficiencyvirusandhavethe[18,19]highefficiencyforinfectionandstablesilencingtargetgene,therefore,itwasusedwidely.210Lentivirus,asgenetransfervector,wascharacterizedbyhightransfectionefficiencyinnon-dividingcellsanddividingcells,washightiter,goodstabilityintargetcellsandlittleimmunoreactivity,andhas[20]beenwidelyusedintransformingintovectorofgeneticengineering.Comparedtoanothercarrierlentiviralvector,ithasitsuniqueadvantages:forsomedifficulttotransfectcellswhichhasahighinfectionrateforprimarycells,stemcellsandundifferentiatedcells,andthegeneticrecombination215couldnotoccurandthevectorcouldbestablyexpressed.Inthisstudy,wefoundthatLXRαgenesweredown-regulatedbyeighty-eightpercentafterlentivirusinfectingbovinemusclesatellitecellsfor48h,theexpressionofABCA1andApoEwerealsodecreased.Meanwhile,LXRαcouldincreasetheexpressionofATP-bindingcassettetransporterprotein-8-
中国科技论文在线http://www.paper.edu.cnandpromotecholesterolefflux.Additionally,afteractivatingLXRαinmacrophages,theexpressionof220ABCA1,ABCG1andApoEalsoincreased.LXRandPPARpathwaycouplingincreasedexpressionof[21]ABCA1andregulatedlipidintakeandreversedtransport.LXRαgenesmightdecreasetheexpressionofApoE.Comparedwiththecontrolgroup,theexpressionofPPARγwasdecreasedinbovinemusclesatellitecells.WhentheexpressionofLXRαgeneisincreased,moreheterodimerscouldbeformedbyPPARγandRXR,inhibitingPPARγandRXRformingheterodimersanddecreasingtheexpressionof[22]225PPARγ.Therefore,whenLXRαwasdown-regulated,LXRαandPPARγcouldformacompetitivemechanism,whichcauseddecreasedexpressionofPPARγ.However,themechanismofup-regulatedexpressionofRXRαhasnotbeenidentifiedandthefurtherstudyshouldberemainedandinvestigated.ItisofgreatsignificancetostudyintracellularlipidmetabolicthroughinterferingLXRαgene.LentivirusinterferingvectorlaysthefoundationforfurtherstudyingLXRαgenefunctioninlipid230metabolisminbovinemusclesatellitecells.4ConclusionlentiviralvectorcarryingtheshRNAtargetingLXRαgenewassuccessfullyconstructed,andthelentiviralvectorcanbeefficientlyexpressedinbovinemusclesatellitecells.WealsoexploredtheeffectsofLXRαgenesilencingonthemetabolicassociatedgenes.Thisstudyprovidesnewinsights235intotheregulationofbovineLXRαincholesterolmetabolism.AcknowledgementsThisworkwassupportedbytheresearchfundforthedoctoralofhighereducationofChina(20130202120008),theChinapostdoctoralsciencefoundation(2015M570811)andthefundamentalresearchfundsforthecentraluniversitiesofChina(GK201502008).240References[1]ThomasPB,LauraAS,WangYJ,etal.Nuclearreceptorsandtheirselectivepharmacologicmodulators[J].PharmacoRev,2013,65:710-778.[2]SavkurRS,Burris,TP.ThecoactivatorLXXLLnuclearreceptorrecognitionmotif[J].TheJournalof245PeptideResearch,2004,63:207-212.[3]ZelcerN,TontonozP.LiverXreceptorsasintegratorsofmetabolicandinflammatorysignaling[J].JClinInvest,2006,116:607-614.[4]LundEG,MenkeJG,SparrowCP.LiverXreceptoragonistsaspotentialtherapeuticagentsfordyslipidemiaandatherosclerosis[J].ArteriosclerThrombVascBiol,2003,23:1169-1177.250[5]OstlundJr.RE.Aminimalmodelforhumanwholebodycholesterolmetabolism[J].AmJPhysiol,1993,265:E513-E520.[6]ZelcerN,TontonozP.LiverXreceptorsasintegratorsofmetabolicandinflammatorysignaling[J].JClinInvest,2006,16(3):607-614.[7]WillyPJ,UmesonoK,OngES,etal.LXR,anuclearreceptorthatdefinesadistinctretinoidresponse255pathway[J].GenesDev,1995,9:1033-1045.[8]VinodM,ChennamsettyI,ColinS,etal.miR-206controlsLXRαexpressionandpromotesLXR-mediatedcholesteroleffluxinmacrophages[J].Biochim.Biophys.Acta,2014,1841(6):827-835.[9]IshimotoK,TachibanaK,SumitomoM,etal.Identificationofhumanlow-densitylipoproteinreceptorasanoveltargetgeneregulatedbyliverXreceptoralpha[J].FebsLett,2006,580:4929-4933.260[10]SoumianS,AlbrechtC,DaviesAH,etal.ABCA1andatherosclerosis[J].VascMed,2005,10:109-119.[11]ChawlaA,BoisvertWA,LeeCH,etal.APPARgamma-LXR-ABCA1pathwayinmacrophagesisinvolvedincholesteroleffluxandathero-genesis[J].MolCell,2001,7(1):161-171.[12]WongJ,QuinnCM,GelissenIC,etal.TheeffectofstatinsonABCA1andABCG1expressioninhumanmacrophagesisinfluencedbycellularcholesterollevelsandextentofdifferentiation[J].Atherosclerosis,2008,265196:180-189.[13]HuYW,MaX,HuangJL,etal.DihydrocapsaicinAttenuatesPlaqueFormationthroughaPPARγ/LXRαPathwayinapoE-/-MiceFedaHigh-Fat/High-CholesterolDiet[J].PLoSOne,2013,8(6):e66876.-9-
中国科技论文在线http://www.paper.edu.cn[14]ZhangY,RepaJY,GauthierK,etal.RegulationofLipoproteinLipasebytheOxysterolReceptors,LXRαandLXRβ[J].JBiolChem,2001,276:43018-43024.270[15]HannonGJ.RNAinterference[J].Nature,2002,418(6894):244-251.[16]SongE,LeeSK,WangJ,etal.RNAinterferencetargetingFasprotectsmicefromfulminanthepatitis[J].NatMed,2003,9:347-351.[16]SongE,LeeSK,WangJ,etal.RNAinterferencetargetingFasprotectsmicefromfulminanthepatitis[J].NatMed,2003,9:347-351.275[17]JacqueJM,TriquesK,StevensonM.ModulationofHIV-1replicationbyRNAinterference[J].Nature,2002,418:435-438.[18]WangS,ZengX,LiuY,etal.ConstructionandcharacterizationofaPDCD5recombinantlentivirusvectoranditsexpressionintumorcells[J].OncolRep,2012,28(1):91-98.[19]ZhaoB,YangC,YangS,etal.Constructionofconditionallentivirus-mediatedshRNAvectortargetingthe280humanMirkgeneandidentificationofRNAiefficiencyinrhabdomyosarcomaRDcells[J].IntJOncol,2013,43(4):1253-1259.[20]KohDM,BrownG,CollinsDJ.Nanoparticlesinrectalcancerimaging[J].CancerBiomark,2009,5(2):89-98.[21]TobinKA,SteinegerHH,AlbertiS,etal.Cross-talkbetweenfattyacidandcholesterolmetabolism285mediatedbyliverXreceptor-alpha[J].MolEndocrinol,2000,14(5):741-752.[22]YoshikawaT,IdeT,ShimanoH,etal.Cross-talkbetweenperoxisomeproliferator-activatedreceptor(PPARα)andliverXreceptor(LXR)innutritionalregulationoffattyacidmetabolismIPPARssuppresssterolregulatoryelementbindingprotein-1cpromoterthroughinhibitionofLXRsignaling[J].MolecularEndocrinology,2003,17(7):1240-1254.290牛LXRα基因mRNA重组慢病毒载体的构建及其对牛肌肉卫星细胞的干扰效果刘永峰,赵璐,库婷,杨兴斌295(食品工程与营养科学学院,陕西师范大学,西安,710062)摘要:肝X受体α(LXRα)基因是核激素受体家族的成员,它可以调节参与胆固醇代谢基因的转录。近期研究发现LXRα被认为是一个调节巨噬细胞内胆固醇平衡的关键因子,从而调节多个基因参与胆固醇运输,如ATP结合蛋白(ABCs)、ABCA1、ABCG1、载脂蛋白E(ApoE)、脂蛋白脂肪酶(LPL)等功能基因。本研究中,以牛LXRα基因为目标基因,设计300了4对shRNA,使用BP和LP重组系统将其构建到慢病毒载体上,在293T细胞中转染获得了相应滴度的载体。Lenti-14MR0054-01、-02、-03和-04四个慢病毒分别以3×108TU/mL、2×108TU/mL、2.5×108TU/mL和3.5×108TU/mL的滴度侵染牛肌肉卫星细胞,定量筛选了最佳载体为pLenti-03,干扰效率达到88%。此外,发现pLenti-03侵染48h后,牛RXRα基因的mRNA表达为上调,同时牛PPARα、PPARγ、ABCA1、LPL和ApoE基因的305mRNA表达为下调。因此,本研究成功构建了高效抑制牛LXRα表达的慢病毒载体,为在牛肌肉卫星细胞中降低LXRα的作用提供了理论基础。关键词:牛LXRα基因;慢病毒;载体构建;基因干扰中图分类号:S858.23-10-'
您可能关注的文档
- 气体稀薄及扩散效应对气凝胶隔热材料传热的影响研究.pdf
- 氧刻蚀超薄碳膜在硬盘保护中的应用.pdf
- 氧化铝对单晶硅表面的纳米磨损研究.pdf
- 氧化锌纳米线的生长、掺杂和应用.pdf
- 水稻品种魔王谷粒形、剑叶性状和株高QTL定位.pdf
- 永磁同步电机转矩波动抑制方法研究.pdf
- 液闪法产氚率测量样品制备中氚逃逸研究.pdf
- 混合能量供应的认知无线电网络中基于效用的协作频谱租借策略研究.pdf
- 牙髓再生治疗术研究新进展.pdf
- 牛传染性鼻气管炎病毒三基因缺失突变株的构建.pdf
- 牛蒡叶提取物对木腐菌抑制能力试验研究.pdf
- 玉米大斑病菌StPP2A-C基因的克隆及原核表达.pdf
- 甜橙中多胺合成酶基因全基因组发掘和表达分析.pdf
- 生长抑制特异蛋白GAS2促进BCR-ABL恶性转化BaF3细胞.pdf
- 电磁轨道炮机电耦合强迫响应研究.pdf
- 电调控La0.5Sr0.5CoO3CeY2Fe5O12氧化物异质结的反射性能.pdf
- 砷的生殖毒性研究.pdf
- 硅质尾矿对发泡水泥性能的影响.pdf
相关文档
- 施工规范CECS140-2002给水排水工程埋地管芯缠丝预应力混凝土管和预应力钢筒混凝土管管道结构设计规程
- 施工规范CECS141-2002给水排水工程埋地钢管管道结构设计规程
- 施工规范CECS142-2002给水排水工程埋地铸铁管管道结构设计规程
- 施工规范CECS143-2002给水排水工程埋地预制混凝土圆形管管道结构设计规程
- 施工规范CECS145-2002给水排水工程埋地矩形管管道结构设计规程
- 施工规范CECS190-2005给水排水工程埋地玻璃纤维增强塑料夹砂管管道结构设计规程
- cecs 140:2002 给水排水工程埋地管芯缠丝预应力混凝土管和预应力钢筒混凝土管管道结构设计规程(含条文说明)
- cecs 141:2002 给水排水工程埋地钢管管道结构设计规程 条文说明
- cecs 140:2002 给水排水工程埋地管芯缠丝预应力混凝土管和预应力钢筒混凝土管管道结构设计规程 条文说明
- cecs 142:2002 给水排水工程埋地铸铁管管道结构设计规程 条文说明