- 859.82 KB
- 2022-04-22 13:45:08 发布
- 1、本文档共5页,可阅读全部内容。
- 2、本文档内容版权归属内容提供方,所产生的收益全部归内容提供方所有。如果您对本文有版权争议,可选择认领,认领后既往收益都归您。
- 3、本文档由用户上传,本站不保证质量和数量令人满意,可能有诸多瑕疵,付费之前,请仔细先通过免费阅读内容等途径辨别内容交易风险。如存在严重挂羊头卖狗肉之情形,可联系本站下载客服投诉处理。
- 文档侵权举报电话:19940600175。
'中国科技论文在线http://www.paper.edu.cnGenome-wideidentificationandexpressionanalysisofthepolyaminebiosynthesisgeneinsweetorange(Citrus5sinensis)WuHao,LiuJihong**(CollegeofHorticultureandForestrySciences,HuazhongAgriculturalUniversity,)Abstract:Polyamines(PAs)arelowmolecularweight,aliphaticpolycationsfoundinthecellsofalllivingorganisms.Andinplants,agrowingnumberofevidencessupportthatPAsplayimportantroles10inabioticstresses.Inthisstudy,atotalof18polyaminebiosynthesisgeneswhichbelongto10kindsofdifferentpolyaminebiosynthesisenzymeswereisolatedfromtheentirecitrusgenomeandafurtheranalysisincludingthechromosomallocations,phylogeneticrelationships,functionalannotations,promoteranalysis,andgenestructureswereperformed.Tissuespecificexpressionofthesegeneswasdetectedinroot,stem,leaf,pulp,peel,andcallus.Thepolyaminebiosynthesisgenedisplayedvarious15responsestoexogenouspolyamines(putrescine,spermidine,spermine)andABAtreatments,andweredifferentiallyalteredbyabioticstresses,includingcoldandsalt.Andthechangepatternsofthreemainpolyaminesduringcoldstressinleavesandcalluswerecharacterized.Thecomprehensiveanalysisofpolyaminebiosynthesisgeneishelpfultoexploitstrategiestoimproveplanttolerancetomultipleenvironmentalstresses.20Keywords:Sweetorange(Citrussinensis);polyaminebiosynthesisgene;abioticstress;Geneexpression0IntroductionPolyamineisanorganiccompoundhavingtwoormoreprimaryaminogroups–NH2.Thereare25threemajorpolyaminesinplants,putrescine(Put),spermidine(Spd),andspermine(Spm)[1],whichplayimportantrolesinvariousprogressesofplantgrowthanddevelopment,includingcellproliferation[2],morphogenesis[3],differentiation[4],programmedcelldeath[5],andresponsestovariousenvironmentalstresses[6-8].Andtheplantstresstolerancewouldbeimprovedwhentreatedwithexogenouspolyamines.Forexample,sprayingputrescinecouldreduce30flooding-inducedoxidativedamagebyincreasingtheantioxidantproperties[9].Exogenousspermidinemaydifferentiallyaltertheactivitiesofsomescavengingsystemenzymes,hydrogenperoxideandsuperoxideradicallevelsinwater-stressedcucumberleaves[10].Applicationofsperminecouldbeexploitedtoalleviateamoderatelevelofosmoticanddehydrationstressthroughtheregulationofstress-relatedcomponentssuchasphotosyntheticpigments,plant35hormones[11]andantioxidants[12].Andalso,overexpressionsomestress-responsegenesmayimproveendogenouspolyaminecontentandobtainahigherstressresistance,likeABF[13,14],ICE1[15],MYB[16],WRKY70[17]andsoon.Throughoneareathatremainslargelyunclearishowpolyamineimprovesplantstressresistance,increasingevidencesshowedthatitmightprotecttheplantsfromstressesbyremovingfreeradicals,maintainingmembraneandcellularstructures,40keepingcation-anionbalance,regulationofionchannelsandinductionofATPsynthesis[1,18-20].Thepolyaminebiosynthesispathway(SupplementFigure1)hasbeeninvestigatedandreviewedcompletelyindetail[21,22].Briefly,putrescinecanbeproduceddirectlyfromL-ornithineBriefauthorintroduction:WuHao(1988-),male,PhDstudent,StressPhysiologyandMolecularBiologyCorrespondanceauthor:LiuJihong(1971-),male,Professor,StressPhsiologyandMolecularbiology.E-mail:liujihong@mail.hzau.edu.cn-1-
中国科技论文在线http://www.paper.edu.cnviaornithinedecarboxylase(ODC,EC4.1.1.17),orindirectlyfromL-arginineviaargininedecarboxylase(ADC,EC4.1.1.19).Spermidineissynthesizedfromputrescinebyspermidine45synthase(SPDS,EC2.5.1.16)withtheadditionofanaminopropylgroupwhichisproducedfromS-adenosylmethionine(SAM)bySAMdecarboxylase(SAMDC,EC4.1.1.50).Thenspermineandthermospermineareproducedwhentheaminopropylgroupisaddedtospermidinebysperminesynthase(SPMS,EC2.5.1.22)andthermosperminesynthase(ACL5,EC2.5.1.79).So,plantpolyaminebiosynthesispathwayincludesfivemainenzymes:ADC,SPDS,SPMS,ACL5and50SAMDC[23,24].ItisworthmentioningthatinArabidopsisthalianaODCpathwaymaybeabsent,indicatingthatputrescineispredominantlyproducedviaADC-mediatedroute.Andoverexpressionorsilenceofdifferentpolyaminebiosynthesisgeneindifferentplantspeciesmaypromoteorweakenstresstolerance[25-28].OverexpressionofPtADCgenemightreduceROScontentandinfluenceonrootgrowthconductivetodroughttolerance[29].Whenoverexpressing55SPDSgeneintomato,theenhancedpolyamineaccumulationcanaltercarotenoidmetabolismatthetranscriptionallevelinfruit[30].CaADC1-silencedpepperhasalowerlevelofpolyaminesandtheexpressionofdefenseresponsegenesweredistinctlylowerinCaADC1-silencedplantsthanthoseintheemptyvectorcontrolplants[31].Underlong-termsalinity,theSPMSandACL5mutantsaccumulatedmoreNa+andperformedworsethanwildtypeinsurvivalexperiments[32].60WhenabrogatetheupstreamopenreadingframeofSAMDC,itwillleadtopolyaminedisruptionandgrowthperturbations[33].Therefore,thereisadeepconnectionbetweenpolyaminebiosynthesisgeneandplanttoleranceofabioticstress.Citrustakesthelargestpartoftheplantareaandhighesttotalyieldintheworld.Butsevereenvironmentalfactorslimitedtheproductionofcitrus,suchascold,drought,salinityandnutrition65deficiency,especiallythecoldstress.Throughthefivedifferentpolyaminebiosynthesisenzymesmayhavedifferentmechanismsofresponsetoabioticstress,agenome-widesurveytofindtherate-limitedenzymeofpolyaminebiosynthesispathwaymayprovideanapproachtoincreasethecitruspolyaminecontentandstresstolerance.Inthisstudy,weidentifiedADC,SPDS,SPMS,ACL5,andSAMDCgenesbystudyingtheirsequencephylogeny,genomeorganization,70chromosomallocation,andcis-actingelements.Therelativeexpressionlevelsofthesegenesindifferenttissuesandvarioustreatment,includingexogenousPAstreatment,stressconditionsandABA,wereexamined.Thepolyaminecontentundercoldstresswasalsodetected.Ourresultsprovideabasicinformationthatmaybeusedforfutureinvestigationintothefunctionsofpolyaminesincitrusdevelopmentandstressresponse.751Results1.1IdentificationofpolyaminebiosynthesisgenefromsweetorangeToidentifypolyaminebiosynthesisgeneinsweetorange,theCitrussinensisAnotationprojectandphytozomewereused,andatotalof18transcriptswereisolatedfromtheentirecitrusgenome-2-
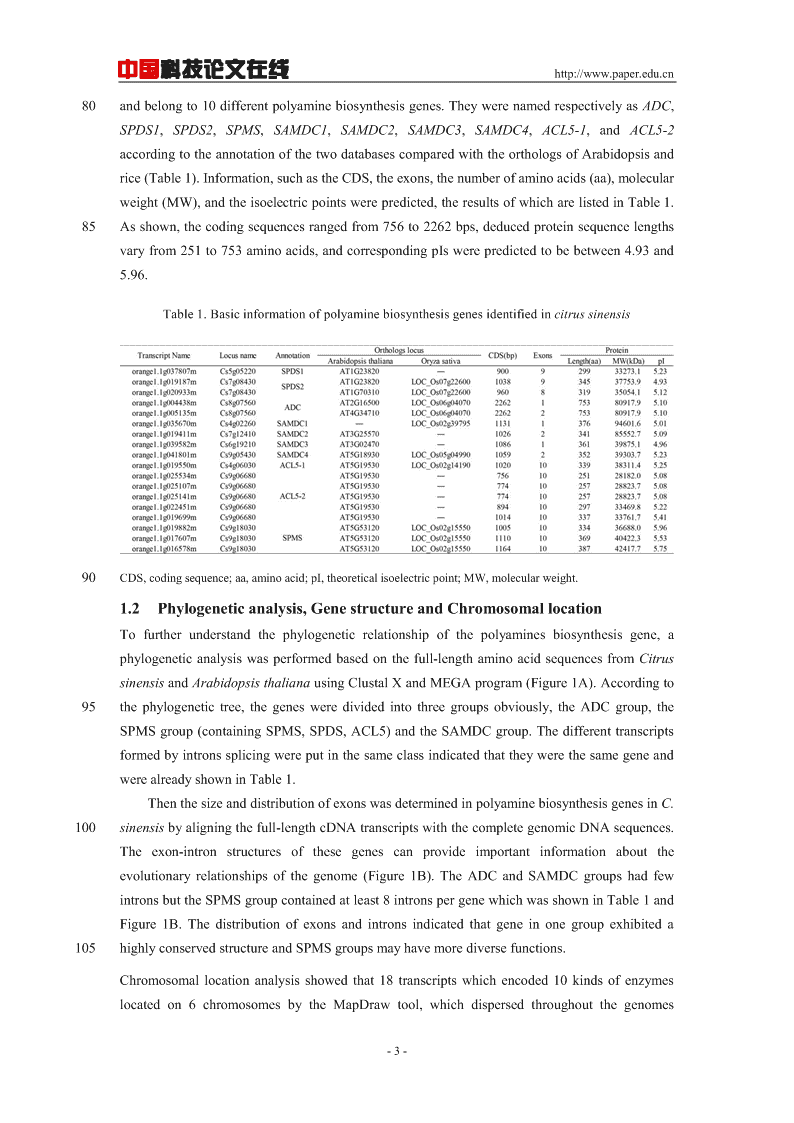
中国科技论文在线http://www.paper.edu.cn80andbelongto10differentpolyaminebiosynthesisgenes.TheywerenamedrespectivelyasADC,SPDS1,SPDS2,SPMS,SAMDC1,SAMDC2,SAMDC3,SAMDC4,ACL5-1,andACL5-2accordingtotheannotationofthetwodatabasescomparedwiththeorthologsofArabidopsisandrice(Table1).Information,suchastheCDS,theexons,thenumberofaminoacids(aa),molecularweight(MW),andtheisoelectricpointswerepredicted,theresultsofwhicharelistedinTable1.85Asshown,thecodingsequencesrangedfrom756to2262bps,deducedproteinsequencelengthsvaryfrom251to753aminoacids,andcorrespondingpIswerepredictedtobebetween4.93and5.96.Table1.Basicinformationofpolyaminebiosynthesisgenesidentifiedincitrussinensis90CDS,codingsequence;aa,aminoacid;pI,theoreticalisoelectricpoint;MW,molecularweight.1.2Phylogeneticanalysis,GenestructureandChromosomallocationTofurtherunderstandthephylogeneticrelationshipofthepolyaminesbiosynthesisgene,aphylogeneticanalysiswasperformedbasedonthefull-lengthaminoacidsequencesfromCitrussinensisandArabidopsisthalianausingClustalXandMEGAprogram(Figure1A).Accordingto95thephylogenetictree,thegenesweredividedintothreegroupsobviously,theADCgroup,theSPMSgroup(containingSPMS,SPDS,ACL5)andtheSAMDCgroup.ThedifferenttranscriptsformedbyintronssplicingwereputinthesameclassindicatedthattheywerethesamegeneandwerealreadyshowninTable1.ThenthesizeanddistributionofexonswasdeterminedinpolyaminebiosynthesisgenesinC.100sinensisbyaligningthefull-lengthcDNAtranscriptswiththecompletegenomicDNAsequences.Theexon-intronstructuresofthesegenescanprovideimportantinformationabouttheevolutionaryrelationshipsofthegenome(Figure1B).TheADCandSAMDCgroupshadfewintronsbuttheSPMSgroupcontainedatleast8intronspergenewhichwasshowninTable1andFigure1B.Thedistributionofexonsandintronsindicatedthatgeneinonegroupexhibiteda105highlyconservedstructureandSPMSgroupsmayhavemorediversefunctions.Chromosomallocationanalysisshowedthat18transcriptswhichencoded10kindsofenzymeslocatedon6chromosomesbytheMapDrawtool,whichdispersedthroughoutthegenomes-3-
中国科技论文在线http://www.paper.edu.cn(Figure1C).TheADCwasonthechromosome8,SPDS1andSPDS2werelocatedonthechromosome5and7respectively,SPMSandACL5geneswasonthechromosome9and110chromosome4.TheSAMDCgeneswerelocatedonchromosome4,7,6and9.Figure1Phylogeneticrelationship,genestructureofcitruspolyaminebiosynthesisgenes.(A)Multiplealignmentof18fulllengthPAbiosynthesisgenesfromcitrusand10fromArabidopsisthalianaweregeneratedbyClustalX2.1.Andthephylogenetictreewasconstructed115inMEGA6.0with1000bootstrapreplicatesusingtheNeighbor-Joiningmethod.(B)Exon/intronorganizationofcitruspolyaminebiosynthesisgenes.Yellowboxesrepresentexons;blacklinesrepresentintronsandpurpleboxesrepresent5’UTR(Un-translatedRegion)and3’UTR.Thesizeofexonsandintronscanbeestimatedusingthescaleatbottom.(C)Chromosomallocationsofcitruspolyaminebiosynthesisgenes.Chrstandsforchromosome.Thelengthofthechromosome120canbeestimatedusingthescaleontheleft.1.3ExpressionpatternsofpolyaminebiosynthesisgenesinresponsetoexogenouspolyaminestreatmentsincitruscallusAccordingtoFigure2B,ADC,SAMDC4,SPDS1andACL5-1weredecreasedunderPuttreatment.TranscriptlevelsofSPDS2andSPMSwereslightlyincreasedat12hand6hbutdecreased125subsequently.TheexpressionlevelofSAMDC1andACL5-2weresignificantlyincreasedat12h,24hand24h,48h,butdecreasedatothertimepoints.SAMDC2exhibitedasimilarexpressionpatternwithSAMDC3whichwasslightlyincreasedatfirstthendecreased.UpontreatmentwithexogenousSpd,theexpressionlevelofADCwasincreasedrapidlyanddecreasedalittleatlast24hours,SPDS2wasincreasedat6hand12handthendecreased130significantly.SAMDC4washighlyup-regulatedat12hand72h.SPMStranscriptlevelwasslightlyincreasedat12h,butdecreasedat48handincreasedagainat72h.TheexpressionpatternsofSAMDC1,SAMDC2,SAMDC3,SPDS2andSPDS3weresimilar.Theywerealldown-regulatedduringexogenousSpdtreatmentandstayedalowerexpressionlevel.AndtheexpressionlevelofACL5-1wasdecreasedat6h,thenreachingthemaximumvalueat12h,but135sharplydecreasedat24hagainandfinallyreachedastablelevelwhichwassamewiththeinitiallevel(Figure2B).TheexpressionpatternsofpolyaminebiosynthesisgenesunderexogenousSpmtreatmentwerequitetheoppositewithSpdtreatment.TheexpressionlevelofADC,SPDS2andSAMDC4-4-
中国科技论文在线http://www.paper.edu.cnremainedaverylowlevelatfirst24hoursandslightlyincreasedat72hafterSpdtreatment.140SPMShadaveryhighexpressionlevelat24hbutdecreasedat48hand72h.SAMDC1,SAMDC2andSAMDC3hadthesameexpressionpatternsthatincreasedsignificantlyat12hand24hthensharplydecreasedat48hand72h.SPDS1stayedaveryhightranscriptlevelexceptat12h.ACL5-2transcriptlevelwasincreasedatfirst12hoursthendecreasedat24handslightlyincreasedagainatlast48hours.TheexpressionlevelofACL5-1underwentnegligiblechanges145afterSpmtreatmentexceptalittledecreasedat24h(Figure2B).1.4ExpressionpatternsofpolyaminebiosynthesisgeneunderabioticstressesandABATofigureouttherolesofthepolyaminebiosynthesisgeneinresponsetodifferentabioticstresses,150andtoexplorewhetherthesegenesparticipateintheABAsignalingpathway,atime-courseexpressionanalysisofthesegenesinresponsetocold(4°C),salinity(NaCl)andABAtreatmentwascarriedout.Undercoldstress,thetranscriptlevelsofADC,SPDS2andACL5-1wererapidlyincreasedat12hbutdecreasedsoon.TheexpressionlevelsofSPDS1,SAMDC3,SAMDC4stayedaveryhigh155levelundercoldstressexceptalittledecreasedat24hinSAMDC4.AndSAMDC1transcriptlevelwasonlyincreasedatlast24hours.ThemRNAlevelsofSPMS,SAMDC2andACL5-2werealldecreasedandkeptaverylowlevelduringcoldstress(Figure2).TheexpressiontrendofADCfollowingsalttreatmentwassimilartothatundercoldstress,beingup-regulatedgenerallyuponexposuretothestress.SPDS1andSPDS2wereslightlydecreasedatfirst24hoursandthen160increasedtothepeakat72h.ThetranscriptlevelofSPMSwasincreasedandACL5-2wasdecreasedalongwiththesalttreatment.SAMDC2andSAMDC3expressionlevelsweredecreasedatfirstthensharplyincreasedat72hand96h.ThetranscriptlevelofSAMDC4wasincreasedtothepeakat12hthendecreasedatlast48hours.SAMDC1andACL5-1sharedasimilarexpressionpatterns,thetranscriptlevelonlyincreasedatlast48hours.165TranscriptlevelsofADCandSPDS1wereremarkablyincreasedat72hand24hseparatelysamewithSAMDC4,butSPDS2,SPMS,SAMDC2andSAMDC3werenotresponsivetoABAastheirmRNAleveldidnotchangenoticeablyfollowingABAtreatment,andevenalittledecreasedcomparedwiththecontrol(0h).ACL5-1andACL5-2sharedthesamepatternsthatthetranscriptlevelbothhadaminorincreaseat12h,24hand72hthendecreasedat72h.Thetranscriptlevel170ofSAMDC1wasnotalteredalotbutdecreasedquicklyat72hsamewithACL5-1andACL5-2.Differentwithcoldandsalttreatment,mostpolyaminebiosynthesisgenesweregenerallynotinducedinABAtreatment(Figure2C).Overall,theexpressionprofilesofthepolyaminebiosynthesisgenesvariedundercold,salinityandABAconditions.-5-
中国科技论文在线http://www.paper.edu.cn175Figure2.TheexpressionpatternsofpolyaminebiosynthesisgeneundertreatmentsofPAsandstressesincitruscallus.(A)Tissue-specificexpressionpatternsofpolyaminebiosynthesisgenes.(B)Theexpressionpatternsofpolyaminesbiosynthesisgenesundertreatmentswithputrescine(Put),spermidine(Spd)andspermine(Spm).(C)Temporal-spatialexpressionpatternsofpolyaminebiosynthesisgenesin180citruscallusundercold,saltandABAtreatment.ThetranscriptlevelsofeachgenewereperformedusingqRT-PCRapproach,andtheresultswerecalculatedusingthe2−∆∆CTmethodwithActinasinternalcontrolgene.Thesamplesthattreatedwith0hweresetascontrol.Thelog2valuesofthecalculatedresultswereusedtogenerateaheatmap.Theintensityvaluebarwasshowedontheleft,andredandgreencolorsrepresenthigherandlowerexpressionlevelsthanthe185control,respectively1.5Cis-actingelementanalysiswithinthepolyaminebiosynthesisgenepromoters.Atotalof62differentkindsofcis-actingelementswereidentifiedinthepromoterregionsofthe190examined10polyaminebiosynthesisgenes(Table2).Themostfrequentoccurrenceofthecis-actingelementisinvolvedinlightresponsiveness.AndManycis-actingelementsinvolvedinstressresponsewerealsorevealed.Forexample,TC-richrepeats(defenseandstressresponse-6-
中国科技论文在线http://www.paper.edu.cnelement)wasfoundinallofthesegenesexceptSAMDC2andSAMDC3,HSE(heatstress-responsiveelement)wasnotfoundonlyinSAMDC3.Similarly,theLTRelement(Low195temperature-responseelement)andDRE/CRTwerealsofoundinallofthesegenesexceptSPDS2.Inaddition,WUN-motif,awound-responsiveelement,wasonlydetectedinSPDS1,SAMDC1andSAMDC3.Meanwhile,severalcis-actingelementsinvolvedinhormoneresponseweredetected.TheABREelementswhosecoresequenceisCGTCinvolvedinABAresponsewerepresentinthe200promoterregionsofallthepolyaminebiosynthesisgenes.GAREmotif,TATC-boxandP-boxinvolvedingibberellinswerefoundinthepromoterregionsofADC,SPDS1,SPDS2,SPMS,SAMDC2,SAMDC4andACL5-1exceptSAMDC1,SAMDC3andACL5-2.TCAelementrelatedtosalicylicacidresponseonlydidnotexistinADC,SAMDC3andSAMDC4promoterregions.CGTCA-motifandTGACG-motif,associatedwithMeJAresponse,existedinSPDS2,SPMS,205SAMDC2,SAMDC4,ACL5-1andACL5-2promoterregions.Incontrast,theauxinresponseelement,AuRR-coreandTGA-elementwereonlyfoundinSPMSandSAMDC4.TheethyleneresponsiveelementwasfoundinSPDS1,SAMDC2,ACL5-1andACL5-2(Table2).Also,somecis-actingelementsrecognizedbytranscriptionfactorswereexistedinthepromoterregions.CACG-motif,recognizedbyNACfamily[20,48],existedinallofthepolyamine210biosynthesisgenes.MBS,recognizedbyMYBfamily[49],alsoexistedinallofthesegenesexceptSAMDC3.W-box,recognizedbyWRKYfamily[50],werefoundinSPDS1,SAMDC2,SAMDC4,ACL5-1,ACL5-2promoterregions.And,ashasbeenmentionedbefore,theDRE/CRT,ABREandEREelementswhichwererecognizedbyCBF,ABFandERFtranscriptionfactorsalsoexistedinthepromoterregionsofpolyaminebiosynthesisgene.What’smore,thereweresomeother215cis-actingelementsexisted.Forexample,AREelement,essentialfortheanaerobicinduction,as-2-box,involvedinshoot-specificexpression,CCGTCC-boxandCAT-box,relatedtomeristemexpression,GCN4-motifandSkn-1,cis-regulatoryelementsinvolvedinendospermexpressionwereallexistedinoneormoregenespromoterregions(Table2).220225230-7-
中国科技论文在线http://www.paper.edu.cnTable2.Cis-actingelementsexistedinthe2kbupstreamregionofpolyaminebiosynthesisgenes.Organismmeansthespeciesthatthecis-actingelementwasfirstidentified.“+”,thesense235strand;“-”,theanti-sensestrand.Andthepositiveornegativesignbehindthenumberrepresentthatthenumberofcis-actingelementsexistedinthesensestrandoranti-sensestrandinthegene’spromoterregion.1.6PolyaminescontentundercodstressincitrusleavesandcallusAccordingtotheFigure3A,thecontentofputrescine(Put)wasmuchhigherthanspermidine(Spd)240andspermine(Spm)incitrusleavesundernormalconditions.AndSpmisalittlebitmorethanSpd.Alongwiththetreatment,thecontentofPutwasincreasedsignificantly.ThePutlevelon36hwasalmost2.5timesthanthecontrol(0h).ButthecontentofSpdshowedanegligibleincrease.AndSpmcontentwasdecreasedlittleatfirst,thenraisedsignificantlybuttheabsolutecontentwasstillmuchlowerthanPut.However,thechangepatternofcallusundercoldstresswasquite245differentwithleaves(Figure3B).Incallus,SpmandSpdcontentweremuchhigherthanPutwhichwascompletelyoppositewiththecontentinleavesundernormalconditions.AndSpmcontentwassignificantlyincreasedat12hbutPutcontentwasdecreasedremarkablyatthesametimepoint.ThentheSpmandPutcontentsreturnedtothenormallevel.AndthecontentofSpdwasslightlyincreasedatfirst12hoursanddecreasedatlast36hours.250-8-
中国科技论文在线http://www.paper.edu.cnFigureChangesofPAlevelsundercoldstressincitrusleafandcallus.BenzoyledfreePAsfrom4-week-oldunboltedplantsthathadbeentreatedwithcoldstressweredetectedbyhigh-pressureliquidchromatography(HPLC).Meanvalues±SDofthreeindependentexperiments255areshown.Asterisksindicatesignificantdifferences(*P<0.05,**P<0.01).2Disscussion2.1CharacterizationofpolyaminebiosynthesisgeneinsweetorangeInthisstudy,atotalof10polyaminebiosynthesisgeneswereidentified.AsshowinTable1,wegotthetranscriptnamesfromphytozomedatabase,thelocusnamefromtheCitrussinensis260Annotationprojectandmadethemone-to-onecorrespondence.Thenumberoftranscriptnameswereabitmorethanthenumberoflocusnamesmeantthatseveraltranscriptssharedasimilarcodingsequences(CDS),theonlydifferentbetweenthemwasthepositionoftheintronswhichwasconfirmedbythegenestructures(Figure1A).InArabidopsis,therearetwogenesencodesargininedecarboxylase,AtADC1andAtADC2[51].Butincitrus,thereisonlyonegenelocatedon265the8chromosomeandnamedCsADC(Cs8g07560)obviously.TherearethreespermidinesynthasesinArabidopsis:AtSPDS1,AtSPDS2andAtSPDS3(alsonamedAtSPMS).ButtheAtSPDS3encodesanovelsperminesynthaseanditisaparalogofpreviouslycharacterizedspermidinesynthases:AtSPDS1andAtSPDS2.Asfarasthespermidinesynthaseandsperminesynthaseincitrus,threegeneswereidentified:Cs5g05220,Cs7g08430andCs9g18030.The270orthologsoffirsttwogenesinArabidopsiswereAT1G23820(AtSPDS1)andAT1G70310(AtSPDS2).So,Cs5g05220andCs7g08430werenamedCsSPDS1andCsSPDS2.Cs9g18030wassupposedtobenamedCsSPDS3,becauseAtSPDS3encodesasperminesynthaseinArabidopsis,soincitrus,itwasnamedtoCsSPMSaccordingtoitspredictedfunction.TheconditionofS-adenosylmethioninedecarboxylasewascomplicated.TherearefourSAMDCgenesin275Arabidopsis,AT3G02470andAT3G25570werebothannotatedtoAtSAMDC,theAT5G15950andAT5G18930werenamedtoAtSAMDC2andAtSAMDC4,andtheAT3G17715wasannotatedtoAtSAMDC3butitwasapseudogene.Soincitrus,wedirectlyusedtheannotationofUniProtKnowledgebase[52].TheCs4g02260,Cs7g12410werenamedtoCsSAMDC1andCsSAMDC2respectively,Cs6g19210wasnamedtoCsSAMDC3sequentially.TheCs9g05430wasnamedto280CsSAMDC4becauseofitsArabidopsisorthologAtSAMDC4.AndCs4g06030andCs9g06680-9-
中国科技论文在线http://www.paper.edu.cnbothwereorthologswithAtACL5(AT5G19530)inArabidopsis.Forconveniencesake,wenamedtheCs4g06030andCs9g06680toCsACL5-1(ThermosperminesynthaseACAULIS5)andCsACL5-2accordingtoitspositiononchromosomes.Inspiteofthis,thisnamingmethodjustprovidesawaytounderstandthefunctionsandmakeaconvenientwaytoresearchthesegenes.285Furtherexperimentsandresearchedshouldbeperformedtoannotatethesegenesaccuratelyandproperly.2.2Thecis-actingelementsandthestressresponsesThepolyaminebiosynthesisgeneshavebeenobservedinresponsetodifferentenvironmentalstress.Forexample,inArabidopsis,ADC1ismainlyinducedbycold[53],whileADC2expression290isstronglyinducedbyseveralabioticstresseslikedehydration,highsalinityandK+deficiency[54,55].AtSAMDC2mRNAwasinducedbysalttreatmenttransientlyat10handinresponsetocoldafter5h.AtSPMSmRNAlevelshowedarapidresponsetosaltstress.Inresponsetocoldtreatment,theAtACL5mRNAappearedafter5h,andincreasedupto24h[56].Generally,stress-inducedgeneshaveacommoncharacteristicthatvariouscis-actingelementsexistedinthe295genepromoterregion.Actually,thecis-actingelementsthatfunctioninstress-responsivegeneexpressionhavebeenpreciselyanalyzedtoelucidatethemolecularmechanismsofgeneexpressioninresponsetostress.Forexample,ABRE[57]isinvolvedindroughtstressandABAsignaltransduction.HSE[58]isinvolvedinheatstress.Thedehydration-responsiveelement(DRE)withthecoresequenceCGACwasidentifiedasacis-actingpromoterelementthatregulatesgene300expressioninresponsetodrought,salt,andcoldstressesinArabidopsis[59].AsimilarmotifwasidentifiedastheC-repeat(CRT)andlow-temperature-responsiveelement(LTR)incoldinduciblegenes[60].Inthisstudy,theqRT-PCRanalysiswasperformedtodetectthegeneexpressionsofpolyaminebiosynthesisgeneunderabioticstresses(Figure2C).Someofthesegenesexpressionlevelswereup-regulatedordown-regulatedundercoldandsalttreatments,butsomewithoutany305change.Forexample,theexpressionlevelofADC,SPDS1andSAMDC4weresignificantlyincreasedandmaintainedahighlevelincitrusleavesduringcoldstress(SupplementFigure3),especiallySAMDC4,whichindicatedthatthesegenesactedaskeymodulatorsinpreventingdamageinsweetorangeasaresultofcold-inducedstresscondition.Andofcourse,theCRTelementwasexistedonthepromoterregionsofthesegenes.Butthereareexceptionsthatthe310transcriptlevelsofSPDS2,SPMS,SAMDC2,SAMDC3andSAMDC4didnotinducedandstayaverylowleveluponABAtreatmentthoughtheABREelementexistedinthesegenespromoterregion(Figure2C,Table2).AndthesamesituationhappenedinSAMDC2andACL5-2undercoldstress.DespitetheCRTelementwasfoundinthepromoterregion,theSAMDC1andACL5-2expressionleveldidnotchangealongwiththecoldtreatment(Figure2C,Table2).315Inaddition,accordingtotheTable2,thepromoterregionsofpolyaminebiosynthesisgenecontainedvariouscis-actingelementsasmentionedabovethatrecognizedbydifferenttranscriptionfactorswhichalsoinvolvedinstressresponse.ButfewstudiesfocusedonthismechanismandmostofthemwererelatedtoADC,therate-limitedenzymeinputrescine-10-
中国科技论文在线http://www.paper.edu.cnbiosynthesis.Forexample,aMYBtranscriptionfactor,PtsrMYB,caninteracttworegionsofthe320ADCpromoterandthetransgenictobaccolinesdisplayedhighermRNAlevelsoftheADCgene[16].AnothertranscriptionfactorWRKY70fromFortunellacrassifoliaalsointeractwiththepromoterofFcADCwhichcontainedtwoW-boxelementsandcanpromoteproductionofputrescineviaregulatingADCexpression.Andrecently,anewNACtranscriptionfactor,PtrNAC72,wasprovedtobindtothepromoterofPtADCandfunctionasarepressorofputrescine325biosynthesis[20].2.3ThepolyaminecontentandpolyaminebiosynthesisgenesRecently,alargenumberofstudiesshowsthatplantpolyaminelevelsareincreasedinresponsetosuchstressesashighandlowtemperatures,salinity,drought,hyperosmosisandhypoxia[8,25].Andpreviousstudyalsorevealedthattheexpressionlevelofpolyaminebiosynthesisgeneswere330inducedindifferentstressconditions.Overexpressionpolyaminebiosynthesisgenesmayelevatetheendogenouspolyaminecontentandimprovethestresstolerance[23,61].InArabidopsis,AtADC2isresponsiblefortheincreaseofADCactivity[62].AndtheenhancedSAMDCexpressionmayleadtothesperminecatabolism[63].Asmentionedabove,inthisstudy,thePutcontentinleaveshadthehighestcontentinsteadofSpmincallusofthethreepolyaminesduringthecold335stress(Figure3).Andcomparedwiththeexpressionpatternsinleavesandcallusduringcoldstress,theputrescinebiosynthesisgeneADCexpressionlevelinleaveswashigherthanthatincalluswhichmayexplainwhythePutcontentwasthehighestinleaves.Butonthecontrary,thesperminebiosynthesisgeneSPMSexpressionlevelincalluswasnotinducedbycoldstresswhichwasoppositewiththehighestcontentofspermineincallus.(Figure2,SupplementFigure3).So340theeffectofpolyaminedegradationmustbetakenintoconsiderationinordertoexplainthisresult.PlantPAOs(polyamineoxidases)catalyzetheoxidationofthepolyaminesubstratesspermidineandspermine.SpdandSpmmaybestronglyoxidizedbyPAOunderthesestressconditions.AndsomeofthePAOswerealsoinducedbyabioticstressesinsweetorange.WangLiu(2015)[64]revealedthatCsPAO4,functionasterminalcatabolismofSpdandSpm,wassignificantlyinduced345attheearlystageofcoldtreatment.Sothegeneexpressionprofileswerenotpreciselycorrelatedwithpolyaminecontents,whichmaybecauseofvariouslevelsofregulationofpolyaminemetabolismasdiscussedabove.3ConclusionPreviousstudieshaveinvestigatedandanalyzedthefeaturesandfunctionsofsomeofthe350polyaminebiosynthesisgeneinvariousspeciessuchasArabidopsis,rice,pepper,cucumber,potatoandLotusjaponicus[27,31,65-68].Tobemoreexact,thisisthefirsttimetoanalysispolyaminebiosynthesisgeneincitrussinensiscomprehensively.Inthisstudy,thetotalof18genesbelongsto10kindsofpolyaminebiosynthesisenzymesbygenome-wideanalysiswereidentified.Thephylogenetictreeandgenestructureweregeneratedwiththefulllengthof355Arabidopsispolyaminebiosynthesisgenesequencestounderstandthepotentialbiological-11-
中国科技论文在线http://www.paper.edu.cnfunctionsbetterduringevolutionaryprocesses.Chromosomallocationanalysesshowedthat18geneswerelocatedon6chromosomesofall9chromosomesincitrus.Theanalysisofpromoterregionsofeachpolyaminebiosynthesisgeneindicatedthatthesegenesmaybeinvolvedinabioticstressesandplanthormonesresponses.Expressionpatternsofthepolyaminebiosynthesisgene360underexogenoustreatmentswithPut,SpdorSpmshowedthatthesegenewereindeedinvolvedinpolyaminebiosynthesisandmetabolism.AndthecontentofthePut,SpdandSpmundernormalgrowthconditionsandcoldstresswasdetectedtofurtherfigureouttheroleofpolyamines.Accordingtotheresultsandpreviousresearches,WeconsideredthattheADCwasoneofthemainrate-limitingenzymesduringcoldstress,wearecurrentlyinvestigatingtheroleofADCin365polyaminebiosynthesisundercoldstressconditionsbyusingtheinteractionsofgenesorproteins.4Materialsandmethods4.1Plantmaterials,growthconditionsandtreatmentsCitruscalluswasculturedonMTmediumandsubculturedeverythreeweeks.Callusgrowthof10daysonthemediumwasusedfortreatment.ThecalluswassubjectedtotreatmentswithPut,Spd,370Spm,abscisicacid(ABA),sodiumchloride(NaCl)andcold(4°C).ForPAstreatment,calluswasculturedat25°CindarkontheMTmediumaddedwith2.0mMPut,1.0mMSpdor2.0mMSpm,followedbysamplingat0,6,12,24,48and72haftertreatment.Forstresstreatments,thecalluswasculturedat25°CinthedarkonMTmediumsupplementedwith100μMABAor100mMNaClrespectively,or4°CindarkonMTmediuminalowtemperatureincubator.Calluswas375collectedat0,12,24,48,72and96hafterthecorrespondingtreatments.Inaddition,samplesatthe0htimepointwereusedascontrolsforallthestressconditions.Toinvestigatetissue-specificexpressionprofiles,roots,stemsandleavesfromsix-week-oldseedlingsofsweetorangewerecollected.Andpulpandpeelswereseparatedfromcitrusfruitspickedinorchards.Allthesampleswerefrozeninliquidnitrogenimmediatelyaftercollected,andstoredat-80℃forfurtheranalysis.380Atleastthreebiologicalreplicateswereperformedforeachsample.4.2IdentificationandSequenceanalysisThepolyaminebiosynthesisgenesequences(includingcodingsequence,transcriptsequenceandgenomesequence)ofArobidopsisthalianaandOryzasativawereobtainedfromtheTAIR[34]and385RiceGenomeAnnotationProject[35].Toidentifythepolyaminebiosynthesisgeneincitrus,thepublishedArabidopsisrelatedgenesasquerysequencesweresubjectedtoatblastnsearchagainsttheCitrussinensisgenomedatabase[36]andthephytozome[37].Tofurthercharacterizethecitruspolyaminebiosynthesisgenes,anonlineExPasyprogram[38]wasusedtocalculatethelengthofaminoacid(aa),molecularweight(Wt),andisoelectricpoint(PI)ofeachprotein.390-12-
中国科技论文在线http://www.paper.edu.cn4.3Phylogeneticanalysis,Genestructure,ChromosomallocationandPromoteranalysisGenestructurepredictionwasgeneratedusingtheonlinewebserverGeneStructureDisplayServer2.0[39].TheresultsweremanuallyadjustedaccordingtotheGT-AGrule.AndtheClustal395Xprogram(version2.1)andGeneDocsoftware(version2.7)wereusedtogeneratemultiplealignmentsoftheproteinsseparatelyaccordingtotheirfunctionsinpolyaminebiosynthesispathway.AndthephylogenetictreesweregeneratedwithMEGAprogram(version6.06)usingtheNeighbor-Joining(NJ)algorithm[40].Bootstrapanalysiswasperformedusing1,000replicatesinMEGAtoevaluatethereliabilityofdifferentphylogeneticgroups[41].MapDraw400tool[42]wasusedtodesignthemapsofphysicallocationofthesegenesandthestartingpositiononeachchromosomewassearchedandconfirmedbyBlastNagainsttheCitrussinensisAnnotationproject[36].Theduplicationeventsofpolyaminebiosynthesisgeneincitrusweresearchedbasedonpreviousparameters:e-value<1e−10andidentity>90%[43].The2000bpupstreamofthestartcodonwastakenastheputativepromoterregionintheCitrussinensis405Annotationproject[36]andsearchedforcis-actingelementsinPlantCAREdatabases[44].4.4RNAisolationandexpressionanalysisTotalRNAswereextractedaccordingtotheinstructionsofTrizolreagent(TaKaRaBioGroup,Japan).ThefirststrandcDNAsweresynthesizedusingPrimeScriptTMPTreagentKitwithgDNA410Eraser(TOYOBOCO.,LTD,Japan).Thegene-specificprimers(supplementalTableS1)weredesignedusingPrimer5.0withmeltingtemperaturesof56-58℃,primerlengthsof19-20bpsandampliconlengthswith100-200bp.Quantitativereal-timePCR(qRT-PCR)wasperformedinAppliedBiosystemsQuantStudio™7FlexReal-TimePCRSystem(ThermoFisherScientific,America)usingtheQuantiNovaTMSYBR®GreenPCRKit(TRANSGENBIOTECH,China).415Eachreactionsystemcontained5μL2×SYBRGreenPCRMasterMix,1μLQNROXReferenceDye,0.35μLofeachprimer,2.3μLwater,andappropriatelydilutedcDNA1μL.Thethermalcyclingconditionswere95°Cfor2minfollowedby40cyclesof95°Cfor5sec,60°Cfor10sec.ThecitrusACTINwasusedastheinternalreferencegene.The2−∆∆CTmethodwasusedtocalculatetheexpressionlevelofdifferentsamples[45].Therepresentativedataareexpressedasthe420meanvalues±standarddeviation(±SD).Eachtreatmentwasrepeatedthriceindependently.TheclusteranalysisandheatmapsweregeneratedusingCluster3.0andTreeviewsoftware.4.5QuantitativeanalysisoffreepolyaminesFrozentissuesweregroundinliquidnitrogenusingamortarandpestle.About0.3gpowderswere425extractedin5%precoolingHCLO4withDTT(0.05g/100ml)basedonthemethoddescribedbyFloresGalston(1982)[46]withmodifications.Sampleswerecentrifugedat12000rpmfor20minafter1hincubationinanicebath.Thesupernatantphase,containingthefreepolyaminefraction,wasstoredfrozenat-20°Cinplasticvials.Derivationandbenzoylationofthepolyamineswere-13-
中国科技论文在线http://www.paper.edu.cnperformedasdescribedbyFu.Chen.Wang,etal.(2011)[47].HPLCanalysiswasperformedwith430aprogrammableAgilentTechnologies1200seriesliquidchromatograph(AgilentTechnologies,US).ThesolventsystemconsistedofHPLC-grademethanol:water,55%:45%(v/v,A:B)to95%:5%in10minataflowrateof0.7ml/min.PAslevels,expressedasnmol/gfreshweight(FW),weretheaverageofthreereplicatesforeachsample.4.6StatisticalAnalysis435Allexperimentaldataareaveragesofatleastthreeindependentreplicates.ThedatawerestatisticallyevaluatedbyapplyingFisher’sLSDtest,intheANOVAprogramofSPSS(IBMSPSSsoftware,version22),takingP<0.05(*)andP<0.01(**)assignificantlydifferent.References[1]YangB.,WuJ.,GaoF.,etal.Polyamine-inducednitricoxidegenerationanditspotential440requirementforperoxideinsuspensioncellsofsoybeancotyledonnodecallus[J].Plantphysiologyandbiochemistry:PPB/Societefrancaisedephysiologievegetale,2014,79:41-47.[2]KimD.W.,WatanabeK.,MurayamaC.,etal.PolyamineOxidase5RegulatesArabidopsisGrowththroughThermospermineOxidaseActivity[J].Plantphysiology,2014,165(4):1575-1590.445[3]FortesA.M.,CostaJ.,SantosF.,etal.ArginineDecarboxylaseexpression,polyaminesbiosynthesisandreactiveoxygenspeciesduringorganogenicnoduleformationinhop[J].Plantsignaling&behavior,2014,6(2):258-269.[4]ConaA.,CenciF.,CervelliM.,etal.Polyamineoxidase,ahydrogenperoxide-producingenzyme,isup-regulatedbylightanddown-regulatedbyauxinintheoutertissuesofthemaize450mesocotyl[J].Plantphysiology,2003,131(2):803-813.[5]YodaH.,YamaguchiY.,SanoH.Inductionofhypersensitivecelldeathbyhydrogenperoxideproducedthroughpolyaminedegradationintobaccoplants[J].Plantphysiology,2003,132(4):1973-1981.[6]SharmaH.S.S.,FlemingC.,SelbyC.,etal.Plantbiostimulants:areviewontheprocessingof455macroalgaeanduseofextractsforcropmanagementtoreduceabioticandbioticstresses[J].Journalofappliedphycology,2013,26(1):465-490.[7]LiuJ.H.,NadaK.,HondaC.,etal.Polyaminebiosynthesisofapplecallusundersaltstress:importanceoftheargininedecarboxylasepathwayinstressresponse[J].Journalofexperimentalbotany,2006,57(11):2589-2599.460[8]LiuJ.H.,KitashibaH.,WangJ.,etal.Polyaminesandtheirabilitytoprovideenvironmentalstresstolerancetoplants[J].PlantBiotechnology,2007,24(1):117-126.[9]YiuJ.C.,JuangL.D.,FangD.Y.T.,etal.Exogenousputrescinereducesflooding-inducedoxidativedamagebyincreasingtheantioxidantpropertiesofWelshonion[J].SciHortic,2009,120(3):306-314.-14-
中国科技论文在线http://www.paper.edu.cn465[10]KubisJ.Exogenousspermidinedifferentiallyaltersactivitiesofsomescavengingsystemenzymes,H(2)O(2)andsuperoxideradicallevelsinwater-stressedcucumberleaves[J].Journalofplantphysiology,2008,165(4):397-406.[11]RadhakrishnanR.,LeeI.-J.SperminePromotesAcclimationtoOsmoticStressbyModifyingAntioxidant,AbscisicAcid,andJasmonicAcidSignalsinSoybean[J].Journalofplant470growthregulation,2012,32(1):22-30.[12]ShiJ.,FuX.Z.,PengT.,etal.Sperminepretreatmentconfersdehydrationtoleranceofcitrusinvitroplantsviamodulationofantioxidativecapacityandstomatalresponse[J].Treephysiology,2010,30(7):914-922.[13]ZhangQ.,WangM.,HuJ.,etal.PtrABFofPoncirustrifoliatafunctionsindehydration475tolerancebyreducingstomataldensityandmaintainingreactiveoxygenspecieshomeostasis[J].Journalofexperimentalbotany,2015,66(19):5911-5927.[14]HuangX.S.,LiuJ.H.,ChenX.J.OverexpressionofPtrABFgene,abZIPtranscriptionfactorisolatedfromPoncirustrifoliata,enhancesdehydrationanddroughttoleranceintobaccoviascavengingROSandmodulatingexpressionofstress-responsivegenes[J].BMCplantbiology,4802010,10:230.[15]HuangX.S.,ZhangQ.,ZhuD.,etal.ICE1ofPoncirustrifoliatafunctionsincoldtolerancebymodulatingpolyaminelevelsthroughinteractingwithargininedecarboxylase[J].Journalofexperimentalbotany,2015,66(11):3259-3274.[16]SunP.,ZhuX.,HuangX.,etal.Overexpressionofastress-responsiveMYBtranscription485factorofPoncirustrifoliataconfersenhanceddehydrationtoleranceandincreasespolyaminebiosynthesis[J].Plantphysiologyandbiochemistry:PPB/Societefrancaisedephysiologievegetale,2014,78:71-79.[17]GongX.,ZhangJ.,HuJ.,etal.FcWRKY70,aWRKYproteinofFortunellacrassifolia,functionsindroughttoleranceandmodulatesputrescinesynthesisbyregulatingarginine490decarboxylasegene[J].Plant,cell&environment,2015,38(11):2248-2262.[18]LopatinA.N.,MakhinaE.N.,NicholsC.G.Potassiumchannelblockbycytoplasmicpolyaminesasthemechanismofintrinsicrectification[J].Nature,1994,372(6504):366-369.[19]KamiabF.,TalaieA.,KhezriM.,etal.Exogenousapplicationoffreepolyaminesenhancesalttoleranceofpistachio(PistaciaveraL.)seedlings[J].PlantGrowthRegulation,2014,72(3):495257-268.[20]WuH.,FuB.,SunP.,etal.ANACtranscriptionfactorrepressesputrescinebiosynthesisandaffectsdroughttolerance[J].Plantphysiology,2016,172(3):1532-1547.[21]MinguetE.G.,Vera-SireraF.,MarinaA.,etal.Evolutionarydiversificationinpolyaminebiosynthesis[J].Molecularbiologyandevolution,2008,25(10):2119-2128.500[22]FuellC.,ElliottK.A.,HanfreyC.C.,etal.Polyaminebiosyntheticdiversityinplantsandalgae[J].Plantphysiologyandbiochemistry:PPB/Societefrancaisedephysiologievegetale,2010,48(7):513-520.-15-
中国科技论文在线http://www.paper.edu.cn[23]ShiH.,ChanZ.Improvementofplantabioticstresstolerancethroughmodulationofthepolyaminepathway[J].Journalofintegrativeplantbiology,2014,56(2):114-121.505[24]LiuJ.H.,WangW.,WuH.,etal.Polyaminesfunctioninstresstolerance:fromsynthesistoregulation[J].Frontiersinplantscience,2015,6:827.[25]GuptaK.,DeyA.,GuptaB.Plantpolyaminesinabioticstressresponses[J].Actaphysiologiaeplantarum/PolishAcademyofSciences,CommitteeofPlantPhysiologyGeneticsandBreeding,2013,35(7):2015-2036.510[26]GuoZ.,TanJ.,ZhuoC.,etal.Abscisicacid,H2O2andnitricoxideinteractionsmediatedcold-inducedS-adenosylmethioninesynthetaseinMedicagosativasubsp.falcatathatconferscoldtolerancethroughup-regulatingpolyamineoxidation[J].Plantbiotechnologyjournal,2014,12(5):601-612.[27]EfroseR.C.,FlemetakisE.,SfichiL.,etal.Characterizationofspermidineandspermine515synthasesinLotusjaponicus:inductionandspatialorganizationofpolyaminebiosynthesisinnitrogenfixingnodules[J].Planta,2008,228(1):37-49.[28]LegockaJ.,KlukA.EffectofsaltandosmoticstressonchangesinpolyaminecontentandargininedecarboxylaseactivityinLupinusluteusseedlings[J].Journalofplantphysiology,2005,162(6):662-668.520[29]WangJ.,SunP.P.,ChenC.L.,etal.AnargininedecarboxylasegenePtADCfromPoncirustrifoliataconfersabioticstresstoleranceandpromotesprimaryrootgrowthinArabidopsis[J].Journalofexperimentalbotany,2011,62(8):2899-2914.[30]NeilyM.H.,MatsukuraC.,MaucourtM.,etal.Enhancedpolyamineaccumulationalterscarotenoidmetabolismatthetranscriptionallevelintomatofruitover-expressingspermidine525synthase[J].Journalofplantphysiology,2011,168(3):242-252.[31]KimN.H.,KimB.S.,HwangB.K.Pepperargininedecarboxylaseisrequiredforpolyamineandgamma-aminobutyricacidsignalingincelldeathanddefenseresponse[J].Plantphysiology,2013,162(4):2067-2083.[32]AletA.I.,SanchezD.H.,CuevasJ.C.,etal.Newinsightsintotheroleofsperminein530Arabidopsisthalianaunderlong-termsaltstress[J].Plantscience:aninternationaljournalofexperimentalplantbiology,2012,182:94-100.[33]HanfreyC.,FranceschettiM.,MayerM.J.,etal.Abrogationofupstreamopenreadingframe-mediatedtranslationalcontrolofaplantS-adenosylmethioninedecarboxylaseresultsinpolyaminedisruptionandgrowthperturbations[J].TheJournalofbiologicalchemistry,2002,535277(46):44131-44139.[34]BerardiniT.Z.,ReiserL.,LiD.,etal.TheArabidopsisinformationresource:Makingandminingthe"goldstandard"annotatedreferenceplantgenome[J].Genesis(NewYork,N.Y.:2000),2015,53(8):474-485.-16-
中国科技论文在线http://www.paper.edu.cn[35]KawaharaY.,delaBastideM.,HamiltonJ.P.,etal.ImprovementoftheOryzasativa540Nipponbarereferencegenomeusingnextgenerationsequenceandopticalmapdata[J].Rice,2013,6(1):4.[36]XuQ.,ChenL.L.,RuanX.,etal.Thedraftgenomeofsweetorange(Citrussinensis)[J].Naturegenetics,2013,45(1):59-66.[37]GoodsteinD.M.,ShuS.,HowsonR.,etal.Phytozome:acomparativeplatformforgreen545plantgenomics[J].Nucleicacidsresearch,2012,40(Databaseissue):D1178-1186.[38]ArtimoP.,JonnalageddaM.,ArnoldK.,etal.ExPASy:SIBbioinformaticsresourceportal[J].Nucleicacidsresearch,2012,40(WebServerissue):W597-603.[39]GuoA.Y.,ZhuQ.H.,ChenX.,etal.GSDS:agenestructuredisplayserver[J].Yichuan=Hereditas/Zhongguoyichuanxuehuibianji,2007,29(8):1023-1026.550[40]TamuraK.,DudleyJ.,NeiM.,etal.MEGA4:MolecularEvolutionaryGeneticsAnalysis(MEGA)softwareversion4.0[J].Molecularbiologyandevolution,2007,24(8):1596-1599.[41]YingS.,ZhangD.F.,LiH.Y.,etal.CloningandcharacterizationofamaizeSnRK2proteinkinasegeneconfersenhancedsalttoleranceintransgenicArabidopsis[J].Plantcellreports,2011,30(9):1683-1699.555[42]LiuR.H.,MengJ.L.MapDraw:amicrosoftexcelmacrofordrawinggeneticlinkagemapsbasedongivengeneticlinkagedata[J].Yichuan=Hereditas/Zhongguoyichuanxuehuibianji,2003,25(3):317-321.[43]SongX.M.,HuangZ.N.,DuanW.K.,etal.Genome-wideanalysisofthebHLHtranscriptionfactorfamilyinChinesecabbage(Brassicarapassp.pekinensis)[J].Molecular560geneticsandgenomics:MGG,2014,289(1):77-91.[44]LescotM.,DehaisP.,ThijsG.,etal.PlantCARE,adatabaseofplantcis-actingregulatoryelementsandaportaltotoolsforinsilicoanalysisofpromotersequences[J].Nucleicacidsresearch,2002,30(1):325-327.[45]LivakK.J.,SchmittgenT.D.Analysisofrelativegeneexpressiondatausingreal-time565quantitativePCRandthe2(-DeltaDeltaC(T))Method[J].Methods(SanDiego,Calif.),2001,25(4):402-408.[46]FloresH.E.,GalstonA.W.Analysisofpolyaminesinhigherplantsbyhighperformanceliquidchromatography[J].Plantphysiology,1982,69(3):701-706.[47]FuX.Z.,ChenC.W.,WangY.,etal.EctopicexpressionofMdSPDS1insweetorange(Citrus570sinensisOsbeck)reducescankersusceptibility:involvementofH2O2productionandtranscriptionalalteration[J].BMCplantbiology,2011,11:55.[48]BuQ.,JiangH.,LiC.B.,etal.RoleoftheArabidopsisthalianaNACtranscriptionfactorsANAC019andANAC055inregulatingjasmonicacid-signaleddefenseresponses[J].Cellresearch,2008,18(7):756-767.-17-
中国科技论文在线http://www.paper.edu.cn575[49]SeoJ.S.,JooJ.,KimM.J.,etal.OsbHLH148,abasichelix-loop-helixprotein,interactswithOsJAZproteinsinajasmonatesignalingpathwayleadingtodroughttoleranceinrice[J].ThePlantjournal:forcellandmolecularbiology,2011,65(6):907-921.[50]JiangY.,LiangG.,YangS.,etal.ArabidopsisWRKY57functionsasanodeofconvergenceforjasmonicacid-andauxin-mediatedsignalinginjasmonicacid-inducedleafsenescence[J].The580Plantcell,2014,26(1):230-245.[51]UranoK.,HoboT.,ShinozakiK.ArabidopsisADCgenesinvolvedinpolyaminebiosynthesisareessentialforseeddevelopment[J].FEBSletters,2005,579(6):1557-1564.[52]UniProtC.UniProt:ahubforproteininformation[J].Nucleicacidsresearch,2015,43(Databaseissue):D204-212.585[53]HummelI.,BourdaisG.,GouesbetG.,etal.DifferentialgeneexpressionofARGININEDECARBOXYLASEADC1andADC2inArabidopsisthaliana:characterizationoftranscriptionalregulationduringseedgerminationandseedlingdevelopment[J].TheNewphytologist,2004,163(3):519-531.[54]Perez-AmadorM.A.,LeonJ.,GreenP.J.,etal.Inductionoftheargininedecarboxylase590ADC2geneprovidesevidencefortheinvolvementofpolyaminesinthewoundresponseinArabidopsis[J].Plantphysiology,2002,130(3):1454-1463.[55]ArmengaudP.,BreitlingR.,AmtmannA.Thepotassium-dependenttranscriptomeofArabidopsisrevealsaprominentroleofjasmonicacidinnutrientsignaling[J].Plantphysiology,2004,136(1):2556-2576.595[56]UranoK.,YoshibaY.,NanjoT.,etal.CharacterizationofArabidopsisgenesinvolvedinbiosynthesisofpolyaminesinabioticstressresponsesanddevelopmentalstages[J].Plant,cell&environment,2003,26(11):1917-1926.[57]SimpsonS.D.,NakashimaK.,NarusakaY.,etal.Twodifferentnovelcis-actingelementsoferd1,aclpAhomologousArabidopsisgenefunctionininductionbydehydrationstressand600dark-inducedsenescence[J].ThePlantjournal:forcellandmolecularbiology,2003,33(2):259-270.[58]HuangY.C.,NiuC.Y.,YangC.R.,etal.Theheat-stressfactorHSFA6bconnectsABAsignalingandABA-mediatedheatresponses[J].Plantphysiology,2016:[59]KizisD.,PagesM.MaizeDRE-bindingproteinsDBF1andDBF2areinvolvedinrab17605regulationthroughthedrought-responsiveelementinanABA-dependentpathway[J].ThePlantjournal:forcellandmolecularbiology,2002,30(6):679-689.[60]JiangC.,IuB.,SinghJ.RequirementofaCCGACcis-actingelementforcoldinductionoftheBN115genefromwinterBrassicanapus[J].Plantmolecularbiology,1996,30(3):679-684.[61]JangS.J.,WiS.J.,ChoiY.J.,etal.Increasedpolyaminebiosynthesisenhancesstress610tolerancebypreventingtheaccumulationofreactiveoxygenspecies:T-DNAmutationalanalysisofOryzasativalysinedecarboxylase-likeprotein1[J].Moleculesandcells,2012,34(3):251-262.-18-
中国科技论文在线http://www.paper.edu.cn[62]SoykaS.,HeyerA.G.ArabidopsisknockoutmutationofADC2generevealsinducibilitybyosmoticstress[J].FEBSletters,1999,458(2):219-223.[63]CuevasJ.C.,Lopez-CobolloR.,AlcazarR.,etal.PutrescineisinvolvedinArabidopsis615freezingtoleranceandcoldacclimationbyregulatingabscisicacidlevelsinresponsetolowtemperature[J].Plantphysiology,2008,148(2):1094-1105.[64]WangW.,LiuJ.H.Genome-wideidentificationandexpressionanalysisofthepolyamineoxidasegenefamilyinsweetorange(Citrussinensis)[J].Gene,2015,555(2):421-429.[65]KubisJ.,Floryszak-WieczorekJ.,Arasimowicz-JelonekM.Polyaminesinduceadaptive620responsesinwaterdeficitstressedcucumberroots[J].Journalofplantresearch,2014,127(1):151-158.[66]SichhartY.,DragerB.ImmunolocalisationofspermidinesynthaseinSolanumtuberosum[J].Phytochemistry,2013,91:117-121.[67]PeremartiA.,BassieL.,YuanD.,etal.Transcriptionalregulationofthericearginine625decarboxylase(Adc1)andS-adenosylmethioninedecarboxylase(Samdc)genesbymethyljasmonate[J].Plantphysiologyandbiochemistry:PPB/Societefrancaisedephysiologievegetale,2010,48(7):553-559.[68]HeweziT.,HoweP.J.,MaierT.R.,etal.ArabidopsisspermidinesynthaseistargetedbyaneffectorproteinofthecystnematodeHeteroderaschachtii[J].Plantphysiology,2010,152(2):630968-984.甜橙中多胺合成酶基因全基因组发掘635和表达分析吴昊,刘继红(华中农业大学园艺林学学院)摘要:多胺是植物的一种生长调节因子,参与植物的生长发育、组织分化、果实成熟与衰老以及抵抗生物和非生物胁迫等重要生理过程,在一定程度内,多胺在细胞中的积累量越640多,其胁迫抗性越强。在本文中共鉴定出了10个主要的多胺合成酶基因,分别为精氨酸脱羧酶ADC,亚精胺合成酶SPDS1,SPDS2,精胺合成酶SPMS,S-腺苷甲硫氨酸脱羧酶SAMDC1,SAMDC2,SAMDC3,SAMDC4,热精胺合成酶ACL5-1和ACL5-2,并对其在不同组织中,不同多胺及胁迫处理向下的表达模式进行了鉴定。关键词:果树学;甜橙;多胺合成酶基因;逆境胁迫;基因表达645中图分类号:请查阅《中国图书馆分类法》-19-'
您可能关注的文档
- 水稻品种魔王谷粒形、剑叶性状和株高QTL定位.pdf
- 永磁同步电机转矩波动抑制方法研究.pdf
- 液闪法产氚率测量样品制备中氚逃逸研究.pdf
- 混合能量供应的认知无线电网络中基于效用的协作频谱租借策略研究.pdf
- 牙髓再生治疗术研究新进展.pdf
- 牛LXRα基因mRNA重组慢病毒载体的构建及其对牛肌肉卫星细胞的干扰效果.pdf
- 牛传染性鼻气管炎病毒三基因缺失突变株的构建.pdf
- 牛蒡叶提取物对木腐菌抑制能力试验研究.pdf
- 玉米大斑病菌StPP2A-C基因的克隆及原核表达.pdf
- 生长抑制特异蛋白GAS2促进BCR-ABL恶性转化BaF3细胞.pdf
- 电磁轨道炮机电耦合强迫响应研究.pdf
- 电调控La0.5Sr0.5CoO3CeY2Fe5O12氧化物异质结的反射性能.pdf
- 砷的生殖毒性研究.pdf
- 硅质尾矿对发泡水泥性能的影响.pdf
- 硫酸镁对放射性脑损伤中NF-κB和ICAM-1表达的影响.pdf
- 纳米图形衬底GaAsSi材料的热应力分布.pdf
- 纳米晶合金高频饱和特性的微磁学模拟.pdf
- 肉桂酸小檗红碱酯的合成及抗炎活性.pdf
相关文档
- 施工规范CECS140-2002给水排水工程埋地管芯缠丝预应力混凝土管和预应力钢筒混凝土管管道结构设计规程
- 施工规范CECS141-2002给水排水工程埋地钢管管道结构设计规程
- 施工规范CECS142-2002给水排水工程埋地铸铁管管道结构设计规程
- 施工规范CECS143-2002给水排水工程埋地预制混凝土圆形管管道结构设计规程
- 施工规范CECS145-2002给水排水工程埋地矩形管管道结构设计规程
- 施工规范CECS190-2005给水排水工程埋地玻璃纤维增强塑料夹砂管管道结构设计规程
- cecs 140:2002 给水排水工程埋地管芯缠丝预应力混凝土管和预应力钢筒混凝土管管道结构设计规程(含条文说明)
- cecs 141:2002 给水排水工程埋地钢管管道结构设计规程 条文说明
- cecs 140:2002 给水排水工程埋地管芯缠丝预应力混凝土管和预应力钢筒混凝土管管道结构设计规程 条文说明
- cecs 142:2002 给水排水工程埋地铸铁管管道结构设计规程 条文说明